Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives
- PMID: 33707948
- PMCID: PMC7943556
- DOI: 10.2147/TCRM.S251668
Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives
Abstract
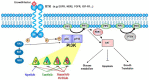
The PI3K/AKT/mTOR pathway has long been known to play a major role in the growth and survival of cancer cells. Breast tumors often harbor PIK3CA gene alterations, which therefore constitute a rational drug target. However, it has taken many years to demonstrate clinically-relevant efficacy of PI3K inhibition and eventually attain regulatory approvals. As data on PI3K inhibitors continue to mature, this review updates and summarizes the current state of the science, including the prognostic role of PIK3CA alterations in breast cancer; the evolution of PI3K inhibitors; the clinical utility of the first-in-class oral selective PI3Kα inhibitor, alpelisib; PIK3CA mutation detection techniques; and adverse effect management. PIK3CA-mutated breast carcinomas predict survival benefit from PI3K inhibitor therapy. The pan-PI3K inhibitor, buparlisib and the beta-isoform-sparing PI3K inhibitor, taselisib, met efficacy endpoints in clinical trials, but pictilisib did not; moreover, poor tolerability of these three drugs abrogated further clinical trials. Alpelisib is better tolerated, with a more manageable toxicity profile; the principal adverse events, hyperglycemia, rash and diarrhea, can be mitigated by intensive monitoring and timely intervention, thereby enabling patients to remain adherent to clinically beneficial treatment. Alpelisib plus endocrine therapy shows promising efficacy for treating postmenopausal women with HR+/HER2- advanced breast cancer. Available evidence supporting using alpelisib after disease progression on first-line endocrine therapy with or without CDK4/6 inhibitors justifies PIK3CA mutation testing upon diagnosing HR+/HER2- advanced breast cancer, which can be done using either tumor tissue or circulating tumor DNA. With appropriate toxicity management and patient selection using validated testing methods, all eligible patients can potentially benefit from this new treatment. Further clinical trials to assess combinations of hormone therapy with PI3K, AKT, mTOR, or CDK 4/6 inhibitors, or studies in men and women with other breast subtypes are ongoing.
Keywords: HR+/HER2− advanced/metastatic breast cancer; PIK3CA mutation test; alpelisib PI3K alpha-selective inhibitor; prognosis; survival benefit; toxicity management.
© 2021 Chang et al.
Conflict of interest statement
Dr Chang reports consultancy/speaker fees from Novartis, Pfizer, AstraZeneca, and Roche, outside the submitted work. Dr Lu discloses clinical study grants/fees and/or consultancy/speaker fees from Novartis, Pfizer, Roche, Eisai, Eli Lily, MSD, and AstraZeneca. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous