Herpes Simplex Virus Type 1 Neuronal Infection Triggers the Disassembly of Key Structural Components of Dendritic Spines
- PMID: 33708072
- PMCID: PMC7940845
- DOI: 10.3389/fncel.2021.580717
Herpes Simplex Virus Type 1 Neuronal Infection Triggers the Disassembly of Key Structural Components of Dendritic Spines
Abstract
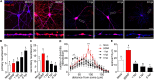
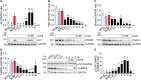
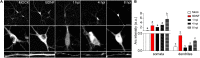
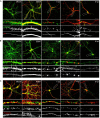
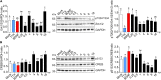
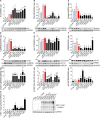
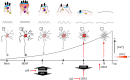
Herpes simplex virus type 1 (HSV-1) is a widespread neurotropic virus. Primary infection of HSV-1 in facial epithelium leads to retrograde axonal transport to the central nervous system (CNS) where it establishes latency. Under stressful conditions, the virus reactivates, and new progeny are transported anterogradely to the primary site of infection. During the late stages of neuronal infection, axonal damage can occur, however, the impact of HSV-1 infection on the morphology and functional integrity of neuronal dendrites during the early stages of infection is unknown. We previously demonstrated that acute HSV-1 infection in neuronal cell lines selectively enhances Arc protein expression - a major regulator of long-term synaptic plasticity and memory consolidation, known for being a protein-interaction hub in the postsynaptic dendritic compartment. Thus, HSV-1 induced Arc expression may alter the functionality of infected neurons and negatively impact dendritic spine dynamics. In this study we demonstrated that HSV-1 infection induces structural disassembly and functional deregulation in cultured cortical neurons, an altered glutamate response, Arc accumulation within the somata, and decreased expression of spine scaffolding-like proteins such as PSD-95, Drebrin and CaMKIIβ. However, whether these alterations are specific to the HSV-1 infection mechanism or reflect a secondary neurodegenerative process remains to be determined.
Keywords: Arc protein; Herpes Simplex Virus Type 1 (HSV-1); dendritic spines; memory consolidation; neurodegeneration; neurotropic virus.
Copyright © 2021 Acuña-Hinrichsen, Covarrubias-Pinto, Ishizuka, Stolzenbach, Martin, Salazar, Castro, Bramham and Otth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

