Impaired Cytoskeletal and Membrane Biophysical Properties of Acanthocytes in Hypobetalipoproteinemia - A Case Study
- PMID: 33708142
- PMCID: PMC7940373
- DOI: 10.3389/fphys.2021.638027
Impaired Cytoskeletal and Membrane Biophysical Properties of Acanthocytes in Hypobetalipoproteinemia - A Case Study
Erratum in
-
Erratum: Impaired Cytoskeletal and Membrane Biophysical Properties of Acanthocytes in Hypobetalipoproteinemia A Case Study.Front Physiol. 2021 May 7;12:695193. doi: 10.3389/fphys.2021.695193. eCollection 2021. Front Physiol. 2021. PMID: 34025461 Free PMC article.
Abstract
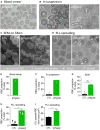
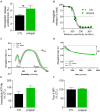
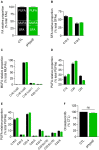
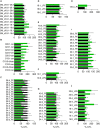
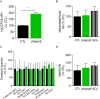
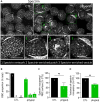
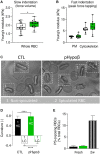
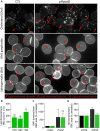
Familial hypobetalipoproteinemia is a metabolic disorder mainly caused by mutations in the apolipoprotein B gene. In its homozygous form it can lead without treatment to severe ophthalmological and neurological manifestations. In contrast, the heterozygous form is generally asymptomatic but associated with a low risk of cardiovascular disease. Acanthocytes or thorny red blood cells (RBCs) are described for both forms of the disease. However, those morphological changes are poorly characterized and their potential consequences for RBC functionality are not understood. Thus, in the present study, we asked whether, to what extent and how acanthocytes from a patient with heterozygous familial hypobetalipoproteinemia could exhibit altered RBC functionality. Acanthocytes represented 50% of the total RBC population and contained mitoTracker-positive surface patches, indicating the presence of mitochondrial fragments. While RBC osmotic fragility, calcium content and ATP homeostasis were preserved, a slight decrease of RBC deformability combined with an increase of intracellular free reactive oxygen species were observed. The spectrin cytoskeleton was altered, showing a lower density and an enrichment in patches. At the membrane level, no obvious modification of the RBC membrane fatty acids nor of the cholesterol content were detected but the ceramide species were all increased. Membrane stiffness and curvature were also increased whereas transversal asymmetry was preserved. In contrast, lateral asymmetry was highly impaired showing: (i) increased abundance and decreased functionality of sphingomyelin-enriched domains; (ii) cholesterol enrichment in spicules; and (iii) ceramide enrichment in patches. We propose that oxidative stress induces cytoskeletal alterations, leading to increased membrane stiffness and curvature and impaired lipid lateral distribution in domains and spicules. In addition, ceramide- and spectrin-enriched patches could result from a RBC maturation defect. Altogether, the data indicate that acanthocytes are associated with cytoskeletal and membrane lipid lateral asymmetry alterations, while deformability is only mildly impaired. In addition, familial hypobetalipoproteinemia might also affect RBC precursors leading to disturbed RBC maturation. This study paves the way for the potential use of membrane biophysics and lipid vital imaging as new methods for diagnosis of RBC disorders.
Keywords: acanthocytosis; ceramide; erythropoiesis; lipid domains; lipidomics; membrane biophysical properties; mitochondria; reactive oxygen species.
Copyright © 2021 Cloos, Daenen, Maja, Stommen, Vanderroost, Van Der Smissen, Rab, Westerink, Mignolet, Larondelle, Terrasi, Muccioli, Dumitru, Alsteens, van Wijk and Tyteca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Red blood cell lipid distribution in the pathophysiology and laboratory evaluation of chorea-acanthocytosis and McLeod syndrome patients.Front Physiol. 2025 Mar 27;16:1543812. doi: 10.3389/fphys.2025.1543812. eCollection 2025. Front Physiol. 2025. PMID: 40213144 Free PMC article.
-
Interplay Between Plasma Membrane Lipid Alteration, Oxidative Stress and Calcium-Based Mechanism for Extracellular Vesicle Biogenesis From Erythrocytes During Blood Storage.Front Physiol. 2020 Jul 3;11:712. doi: 10.3389/fphys.2020.00712. eCollection 2020. Front Physiol. 2020. PMID: 32719614 Free PMC article.
-
Aberrant Membrane Composition and Biophysical Properties Impair Erythrocyte Morphology and Functionality in Elliptocytosis.Biomolecules. 2020 Jul 29;10(8):1120. doi: 10.3390/biom10081120. Biomolecules. 2020. PMID: 32751168 Free PMC article.
-
Structural and mechanical properties of the red blood cell's cytoplasmic membrane seen through the lens of biophysics.Front Physiol. 2022 Sep 12;13:953257. doi: 10.3389/fphys.2022.953257. eCollection 2022. Front Physiol. 2022. PMID: 36171967 Free PMC article. Review.
-
Squeezing for Life - Properties of Red Blood Cell Deformability.Front Physiol. 2018 Jun 1;9:656. doi: 10.3389/fphys.2018.00656. eCollection 2018. Front Physiol. 2018. PMID: 29910743 Free PMC article. Review.
Cited by
-
Lauric Acid, a Dietary Saturated Medium-Chain Fatty Acid, Elicits Calcium-Dependent Eryptosis.Cells. 2021 Dec 1;10(12):3388. doi: 10.3390/cells10123388. Cells. 2021. PMID: 34943896 Free PMC article.
-
Splenectomy improves erythrocyte functionality in spherocytosis based on septin abundance, but not maturation defects.Blood Adv. 2023 Sep 12;7(17):4705-4720. doi: 10.1182/bloodadvances.2022009114. Blood Adv. 2023. PMID: 36753606 Free PMC article.
-
Analysis of Brain, Blood, and Testis Phenotypes Lacking the Vps13a Gene in C57BL/6N Mice.Int J Mol Sci. 2024 Jul 16;25(14):7776. doi: 10.3390/ijms25147776. Int J Mol Sci. 2024. PMID: 39063018 Free PMC article.
-
Phosphatidylethanolamines are the Main Lipid Class Altered in Red Blood Cells from Patients with VPS13A Disease/Chorea-Acanthocytosis.Mov Disord. 2025 Mar;40(3):544-549. doi: 10.1002/mds.30086. Epub 2024 Dec 12. Mov Disord. 2025. PMID: 39665525 Free PMC article.
-
Red blood cell lipid distribution in the pathophysiology and laboratory evaluation of chorea-acanthocytosis and McLeod syndrome patients.Front Physiol. 2025 Mar 27;16:1543812. doi: 10.3389/fphys.2025.1543812. eCollection 2025. Front Physiol. 2025. PMID: 40213144 Free PMC article.
References
-
- Biemer J. J. (1980). Acanthocytosis–biochemical and physiological considerations. Ann. Clin. Lab. Sci. 10 238–249. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

