Retrospective Analysis of Checkpoint Inhibitor Therapy-Associated Cases of Bullous Pemphigoid From Six German Dermatology Centers
- PMID: 33708189
- PMCID: PMC7940359
- DOI: 10.3389/fimmu.2020.588582
Retrospective Analysis of Checkpoint Inhibitor Therapy-Associated Cases of Bullous Pemphigoid From Six German Dermatology Centers
Abstract
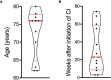
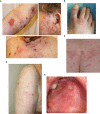
Immune-related adverse events (irAEs) are a class-effect of checkpoint inhibitors (CIs). The development of a Bullous pemphigoid (BP)-like blistering disease, driven by autoantibodies against the hemidesmosomal protein BP180, is a potentially serious irAE whose incidence seems to be increasing. We therefore set out to characterize the clinical and (immuno)histopathological features and treatment responses of cases of BP which developed during or after CI therapy collated in six German tertiary referral centers between 2014 and 2018. We identified twelve cases of BP which emerged during and/or after CI therapy. The time interval between the initiation of CI therapy and the diagnosis of BP was 3-74 weeks (median: 23 weeks). Age at the time of diagnosis of BP varied between 62 and 80 years (median: 76 years). The clinical presentation of the patients was diverse but the severity was relatively mild when compared to that seen in most cases of spontaneous BP. Only four patients met all of the immunopathological criteria recommended in the European guidelines for the diagnosis of BP. Topical corticosteroid treatment was sufficient to achieve disease control in most patients. CI therapy could be continued in 8 out of 12 patients. In summary, our study indicates that cases of BP during or after CI therapy bear several peculiarities distinguishing them from spontaneous BP. Given the diversity of the clinical presentation of CI-induced BP the application of existing diagnostic algorithms developed for spontaneous BP can be utilized to uncover the frequency and features of CI-induced BP and to develop and optimize management algorithms.
Keywords: PD-1 - PD-L1 axis; autoantibodies; autoimmunity; checkpoint inhibitors; ipilimumab; nivolumab; pembrolizumab; pemphigoid disease.
Copyright © 2021 Sadik, Langan, Gutzmer, Fleischer, Loquai, Reinhardt, Meier, Göppner, Herbst, Zillikens and Terheyden.
Conflict of interest statement
DG reports personal fees and other from Pierre Fabre Pharma Gmbh, personal fees and other from Roche Pharma AG, personal fees and other from Bristol-Myers Squibb GmbH & Co. KGaA, personal fees and other from Novartis Pharma GmbH, personal fees and other from Sanofi-Aventis GmbH, during the conduct of the study; other from Amgen GmbH, other from Janssen-Cilag GmbH, other from Galderma Laberatorium GmbH, outside the submitted work. RG reports personal fees and non-financial support from BristolMyersSquibb, personal fees and non-financial support from Roche Pharma, grants, personal fees and non-financial support from Merck Serono, grants, personal fees and non-financial support from Amgen, personal fees and non-financial support from Pierre Fabre, grants, personal fees and non-financial support from Sanofi Regeneron, personal fees from MerckSharpDohme, grants, personal fees and non-financial support from Novartis, personal fees from Almirall Hermal, grants and personal fees from Pfizer, personal fees from SUN Pharma, personal fees from 4SC, grants from Johnson&Johnson outside the submitted work. RH reports personal fees from ROCHE, personal fees from BMS, personal fees from MSD, personal fees from Novartis, personal fees from Pierre-Fabre, outside the submitted work. EL reports personal fees and non-financial support from BristolMyersSquibb, personal fees and non-financial support from Novartis, Meeting and travel support from Curevac and advisory board fees from Sun Pharma. CL reports personal fees from BMS, personal fees from MSD, personal fees from Sanofi, personal fees from Novartis, personal fees from Roche, personal fees from Pierre Fabre, personal fees from Amgen, personal fees from Kyowa Kirin, personal fees from Biontech, personal fees from Almiral Hermal, personal fees from Sun Pharma, personal fees from Merck, outside the submitted work. PT: speaker´s honoraria from BMS, Novartis, MSD, Pierre-Fabre, CureVac and Roche, consultant´s honoraria from BMS, Novartis, Pierre-Fabre, Merck Serono, Sanofi und Roche and travel support fom BMS, Pierre-Fabre and Roche. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Checkpoint inhibitor-associated bullous cutaneous immune-related adverse events: a multicentre observational study.Br J Dermatol. 2022 Dec;187(6):981-987. doi: 10.1111/bjd.21836. Epub 2022 Sep 6. Br J Dermatol. 2022. PMID: 35976170
-
Association of Bullous Pemphigoid With Immune Checkpoint Inhibitor Therapy in Patients With Cancer: A Systematic Review.JAMA Dermatol. 2022 Aug 1;158(8):933-941. doi: 10.1001/jamadermatol.2022.1624. JAMA Dermatol. 2022. PMID: 35612829
-
A Case of Nivolumab-Induced Bullous Pemphigoid: Review of Dermatologic Toxicity Associated with Programmed Cell Death Protein-1/Programmed Death Ligand-1 Inhibitors and Recommendations for Diagnosis and Management.Oncologist. 2018 Oct;23(10):1119-1126. doi: 10.1634/theoncologist.2018-0128. Epub 2018 Jul 17. Oncologist. 2018. PMID: 30018132 Free PMC article. Review.
-
A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors.Int J Dermatol. 2018 Jun;57(6):664-669. doi: 10.1111/ijd.13984. Epub 2018 Apr 6. Int J Dermatol. 2018. PMID: 29630716 Review.
-
Checkpoint Inhibition May Trigger the Rare Variant of Anti-LAD-1 IgG-Positive, Anti-BP180 NC16A IgG-Negative Bullous Pemphigoid.Front Immunol. 2019 Aug 14;10:1934. doi: 10.3389/fimmu.2019.01934. eCollection 2019. Front Immunol. 2019. PMID: 31474998 Free PMC article.
Cited by
-
Pemphigoid diseases as immune-related adverse effect following immune checkpoint inhibitors: A clinical case series of a diverse spectrum.JAAD Case Rep. 2025 Apr 4;61:100-106. doi: 10.1016/j.jdcr.2025.03.021. eCollection 2025 Jul. JAAD Case Rep. 2025. PMID: 40538786 Free PMC article. No abstract available.
-
PD-L1 expression in keratinocyte and infiltration of CD4 + T lymphocyte can predict a severe type of erythema multiforme major induced by the anti-PD-1 antibody, pembrolizumab.Int Cancer Conf J. 2024 Mar 29;13(3):268-274. doi: 10.1007/s13691-024-00676-4. eCollection 2024 Jul. Int Cancer Conf J. 2024. PMID: 38962048 Free PMC article.
-
Analysis of the clinical characteristics of pembrolizumab-induced bullous pemphigoid.Front Oncol. 2023 Mar 3;13:1095694. doi: 10.3389/fonc.2023.1095694. eCollection 2023. Front Oncol. 2023. PMID: 36937423 Free PMC article.
-
Drug eruptions with novel targeted therapies - immune checkpoint and EGFR inhibitors.J Dtsch Dermatol Ges. 2021 Nov;19(11):1621-1643. doi: 10.1111/ddg.14641. J Dtsch Dermatol Ges. 2021. PMID: 34811916 Free PMC article.
-
Targeting type 2 inflammation in bullous pemphigoid: current and emerging therapeutic approaches.Front Med (Lausanne). 2023 Aug 8;10:1196946. doi: 10.3389/fmed.2023.1196946. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37614956 Free PMC article. Review.
References
-
- Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F, Jr., Sznol M, et al. . Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol (2018) 79(6):1081–8. 10.1016/j.jaad.2018.07.008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

