CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance
- PMID: 33708195
- PMCID: PMC7940844
- DOI: 10.3389/fimmu.2021.590054
CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance
Abstract
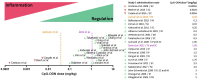
Prevalence and incidence of IgE-mediated allergic diseases have increased over the past years in developed and developing countries. Allergen-specific immunotherapy (AIT) is currently the only curative treatment available for allergic diseases that has long-term efficacy. Although AIT has been proven successful as an immunomodulatory therapy since its beginnings, it still faces several unmet needs and challenges today. For instance, some patients can experience severe side effects, others are non-responders, and prolonged treatment schedules can lead to lack of patient adherence and therapy discontinuation. A common strategy to improve AIT relies on the use of adjuvants and immune modulators to boost its effects and improve its safety. Among the adjuvants tested for their clinical efficacy, CpG oligodeoxynucleotide (CpG-ODN) was investigated with limited success and without reaching phase III trials for clinical allergy treatment. However, recently discovered immune tolerance-promoting properties of CpG-ODN place this adjuvant again in a prominent position as an immune modulator for the treatment of allergic diseases. Indeed, it has been shown that the CpG-ODN dose and concentration are crucial in promoting immune regulation through the recruitment of pDCs. While low doses induce an inflammatory response, high doses of CpG-ODN trigger a tolerogenic response that can reverse a pre-established allergic milieu. Consistently, CpG-ODN has also been found to stimulate IL-10 producing B cells, so-called B regulatory cells (Bregs). Accordingly, CpG-ODN has shown its capacity to prevent and revert allergic reactions in several animal models showing its potential as both preventive and active treatment for IgE-mediated allergy. In this review, we describe how CpG-ODN-based therapies for allergic diseases, despite having shown limited success in the past, can still be exploited further as an adjuvant or immune modulator in the context of AIT and deserves additional attention. Here, we discuss the past and current knowledge, which highlights CpG-ODN as a potential adjuvant to be reevaluated for the enhancement of AIT when used in appropriate conditions and formulations.
Keywords: CpG-oligonucleotides; allergen-specific immunotherapy; allergy; immune tolerance; tolerance induction.
Copyright © 2021 Montamat, Leonard, Poli, Klimek and Ollert.
Conflict of interest statement
MO reports personal fees from Allergy Therapeutics/Bencard, Great Britain/Germany; Thermo Fisher Scientific, Sweden; Siemens Healthcare Diagnostics, Germany; Hitachi Chemical Diagnostics, USA; and Hycor Diagnostics, USA outside the submitted work; and is Scientific co-founder of the academic biotech spin-offs PLS-Design GmbH, Hamburg, Germany and Tolerogenics SARL, Luxembourg. CL and MO report to be co-inventors on two patents pending on hydrogel-embedded oligodeoxynucleotides as tolerogenic adjuvant for subcutaneous immunotherapy and induction of allergen-specific Tregs prior to oral or sublingual immunotherapy of food allergy. LK reports receiving research grants from Allergy Therapeutics/Bencard, Great Britain/Germany; ALK-Abelló, Denmark; Allergopharma, Germany; ASIT Biotech, Belgium; AstraZeneca, Sweden, Biomay, Austria, Boehringer Ingelheim, Germany, Circassia, USA; Stallergenes, France; Cytos, Switzerland; Curalogic, Denmark; HAL, Netherlands; Lofarma, Italy; Mylan, USA; Novartis, Switzerland, Leti, Spain; ROXALL, Germany; GlaxoSmithKline (GSK), Great Britain; Sanofi, France and/or has served on the speaker’s bureau or was consulting for the above mentioned pharmaceutical companies. LK is the current President of AeDA (German Society of Applied Allergology), Vice-President of the German Academy for Allergy and Environmental Diseases and Chair of EAACI ENT section. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

