Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer
- PMID: 33708198
- PMCID: PMC7940508
- DOI: 10.3389/fimmu.2021.595722
Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer
Abstract
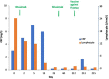
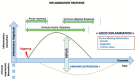
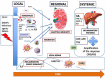
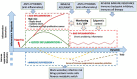
Normal or "good" inflammation process starts from a local cellular response against injury or any infectious agent, with the activation of neutrophils, macrophages, Langerhans cells, dendritic cells, and innate immune cells. Cytokines and chemokines are produced to amplify the local inflammatory process followed by the migration of immune cells to the regional lymph nodes where adaptive immune response is initiated. Systemic inflammation enhances the biological response to mobilize additional cells from central and peripheral immune/hematopoietic system. Local mechanisms to limit inflammation are initiated and lead to healing. During the normal inflammatory process, there is a balance between the production of inflammatory chemokines/cytokines such as Tumor Necrosis Factor (TNF)-α, interleukin (IL)-6 and IL-1 and the production of compounds that limit inflammation and have an immune suppressive effect, such as IL-10 and Transforming Factor (TGF) β. IL-6 and IL-6/soluble IL-6 Receptor (R) complex stimulate liver cells to produce inflammatory proteins, which represents the systemic inflammation response. The magnitude and the duration of the systemic inflammatory response are linked to the cause, under genetic and epigenetic control. Significant inflammation as seen in septic shock, in severe forms of infections or in certain active cancers, represents the "bad inflammation", correlated with a poor prognosis. In addition, the persistence of a chronic smoldering inflammation may lead to pathological situations which are observed in the majority of inflammatory, degenerative, dysmetabolic, or dysimmune diseases and cancer. Chronic smoldering inflammation is a cross between different pathological situations possibly linked. In addition, within the tumor microenvironment, inflammatory process results from different cellular mechanisms modulated by metabolic and vascular changes. On the contrary, a limited and balanced inflammation initiates the normal immune response, including the adaptive response which amplifies any immunotherapy, including vaccines. Immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cells are associated with cytokine release syndrome, a clinical risk leading to the use of anti-cytokine drugs. Nowadays, it is time to monitor the dynamic inflammatory process for a better immune precision medicine in both infections and cancer.
Keywords: cancer; immune therapy; infection; inflammation; precision medicine.
Copyright © 2021 Rossi, Lu, Massart and Levon.
Conflict of interest statement
J-FR, KL, and CM are co-founders of E-Sana. J-FR is consultant for Leo Pharma, NPO Petrovax and Eusapharma. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Burny W, Marchant A, Hervé C, Callegaro A, Caubet M, Fissette L, et al. . ECR-008 study group. Inflammatory parameters associated with systemic reactogenicity following vaccination with adjuvanted hepatitis B vaccines in humans. Vaccine (2019) 37:2004–15. 10.1016/j.vaccine.2019.02.015 - DOI - PubMed
-
- Weiner J, Lewis DJM, Maertzdorf J, Mollenkopf HJ, Bodinham C, Pizzoferro K, et al. . Characterization of potential biomarkers of reactogenicity of licensed antiviral vaccines: randomized controlled clinical trials conducted by the BIOVACSAFE consortium. Sci Rep (2019) 9:20362. 10.1038/s41598-019-56994-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

