Immunopathogenesis of Craniotomy Infection and Niche-Specific Immune Responses to Biofilm
- PMID: 33708216
- PMCID: PMC7940520
- DOI: 10.3389/fimmu.2021.625467
Immunopathogenesis of Craniotomy Infection and Niche-Specific Immune Responses to Biofilm
Abstract
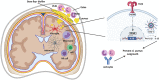
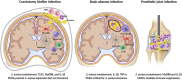
Bacterial infections in the central nervous system (CNS) can be life threatening and often impair neurological function. Biofilm infection is a complication following craniotomy, a neurosurgical procedure that involves the removal and replacement of a skull fragment (bone flap) to access the brain for surgical intervention. The incidence of infection following craniotomy ranges from 1% to 3% with approximately half caused by Staphylococcus aureus (S. aureus). These infections present a significant therapeutic challenge due to the antibiotic tolerance of biofilm and unique immune properties of the CNS. Previous studies have revealed a critical role for innate immune responses during S. aureus craniotomy infection. Experiments using knockout mouse models have highlighted the importance of the pattern recognition receptor Toll-like receptor 2 (TLR2) and its adaptor protein MyD88 for preventing S. aureus outgrowth during craniotomy biofilm infection. However, neither molecule affected bacterial burden in a mouse model of S. aureus brain abscess highlighting the distinctions between immune regulation of biofilm vs. planktonic infection in the CNS. Furthermore, the immune responses elicited during S. aureus craniotomy infection are distinct from biofilm infection in the periphery, emphasizing the critical role for niche-specific factors in dictating S. aureus biofilm-leukocyte crosstalk. In this review, we discuss the current knowledge concerning innate immunity to S. aureus craniotomy biofilm infection, compare this to S. aureus biofilm infection in the periphery, and discuss the importance of anatomical location in dictating how biofilm influences inflammatory responses and its impact on bacterial clearance.
Keywords: Staphylococcus aureus; biofilm; craniotomy; macrophage; microglia; myeloid-derived suppressor cell; neutrophil.
Copyright © 2021 de Morais, Kak, Menousek and Kielian.
Conflict of interest statement
A patent has been filed with the US Patent and Trademark Office covering the application of 3D bioprinted scaffolds for the treatment of craniotomy-associated infections that is discussed in this review (PCT/US2020/021440; TK). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Fernandez-de Thomas RJ, De Jesus O. Craniotomy. Treasure Island (FL): StatPearls; (2020).
-
- Pinto VL, Tadi P, Adeyinka A. Increased Intracranial Pressure. Treasure Island (FL): StatPearls; (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

