Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO
- PMID: 33708223
- PMCID: PMC7940516
- DOI: 10.3389/fimmu.2021.636081
Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO
Abstract
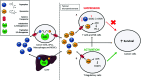
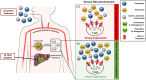
Blockade of the immunosuppressive tryptophan catabolism mediated by indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO) holds enormous promise for sensitising cancer patients to immune checkpoint blockade. Yet, only IDO1 inhibitors had entered clinical trials so far, and those agents have generated disappointing clinical results. Improved understanding of molecular mechanisms involved in the immune-regulatory function of the tryptophan catabolism is likely to optimise therapeutic strategies to block this pathway. The immunosuppressive role of tryptophan metabolite kynurenine is becoming increasingly clear, but it remains a mystery if tryptophan exerts functions beyond serving as a precursor for kynurenine. Here we hypothesise that tryptophan acts as a rheostat of kynurenine-mediated immunosuppression by competing with kynurenine for entry into immune T-cells through the amino acid transporter called System L. This hypothesis stems from the observations that elevated tryptophan levels in TDO-knockout mice relieve immunosuppression instigated by IDO1, and that the vacancy of System L transporter modulates kynurenine entry into CD4+ T-cells. This hypothesis has two potential therapeutic implications. Firstly, potent TDO inhibitors are expected to indirectly inhibit IDO1 hence development of TDO-selective inhibitors appears advantageous compared to IDO1-selective and dual IDO1/TDO inhibitors. Secondly, oral supplementation with System L substrates such as leucine represents a novel potential therapeutic modality to restrain the immunosuppressive kynurenine and restore anti-tumour immunity.
Keywords: AhR; IDO1; System L; TDO; immunotherapy; inhibitors; kynurenine; tryptophan.
Copyright © 2021 Kim and Tomek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A highly efficient modality to block the degradation of tryptophan for cancer immunotherapy: locked nucleic acid-modified antisense oligonucleotides to inhibit human indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase expression.Cancer Immunol Immunother. 2020 Jan;69(1):57-67. doi: 10.1007/s00262-019-02438-1. Epub 2019 Dec 4. Cancer Immunol Immunother. 2020. PMID: 31802183 Free PMC article.
-
4,5-Disubstituted 1,2,3-triazoles: Effective Inhibition of Indoleamine 2,3-Dioxygenase 1 Enzyme Regulates T cell Activity and Mitigates Tumor Growth.Sci Rep. 2019 Dec 5;9(1):18455. doi: 10.1038/s41598-019-54963-9. Sci Rep. 2019. PMID: 31804586 Free PMC article.
-
Editorial: Targeting Indoleamine 2,3-dioxygenases and Tryptophan Dioxygenase for Cancer Immunotherapy.Front Immunol. 2021 Dec 6;12:789473. doi: 10.3389/fimmu.2021.789473. eCollection 2021. Front Immunol. 2021. PMID: 34938297 Free PMC article. No abstract available.
-
Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy.Clin Cancer Res. 2015 Dec 15;21(24):5427-33. doi: 10.1158/1078-0432.CCR-15-0420. Epub 2015 Oct 30. Clin Cancer Res. 2015. PMID: 26519060 Free PMC article. Review.
-
Targeting key dioxygenases in tryptophan-kynurenine metabolism for immunomodulation and cancer chemotherapy.Drug Discov Today. 2015 May;20(5):609-17. doi: 10.1016/j.drudis.2014.11.007. Epub 2014 Dec 3. Drug Discov Today. 2015. PMID: 25478733 Review.
Cited by
-
Metabolic Barriers to Glioblastoma Immunotherapy.Cancers (Basel). 2023 Feb 28;15(5):1519. doi: 10.3390/cancers15051519. Cancers (Basel). 2023. PMID: 36900311 Free PMC article. Review.
-
Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis.Gut. 2023 Nov 24;72(12):2272-2285. doi: 10.1136/gutjnl-2023-329543. Gut. 2023. PMID: 37770127 Free PMC article.
-
Surface-Enhanced Raman Scattering Monitoring of Tryptophan Dynamics in 3D Pancreatic Tumor Models.ACS Sens. 2024 Aug 23;9(8):4236-4247. doi: 10.1021/acssensors.4c01210. Epub 2024 Jul 22. ACS Sens. 2024. PMID: 39038809 Free PMC article.
-
Kynureninase Upregulation Is a Prominent Feature of NFR2-Activated Cancers and Is Associated with Tumor Immunosuppression and Poor Prognosis.Cancers (Basel). 2023 Jan 29;15(3):834. doi: 10.3390/cancers15030834. Cancers (Basel). 2023. PMID: 36765792 Free PMC article.
-
Laeverin/aminopeptidase Q induces indoleamine 2,3-dioxygenase-1 in human monocytes.iScience. 2023 Aug 19;26(9):107692. doi: 10.1016/j.isci.2023.107692. eCollection 2023 Sep 15. iScience. 2023. PMID: 37705960 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

