Adult human liver slice cultures: Modelling of liver fibrosis and evaluation of new anti-fibrotic drugs
- PMID: 33708350
- PMCID: PMC7934011
- DOI: 10.4254/wjh.v13.i2.187
Adult human liver slice cultures: Modelling of liver fibrosis and evaluation of new anti-fibrotic drugs
Abstract
Background: Liver fibrosis can result in end-stage liver failure and death.
Aim: To examine human liver fibrogenesis and anti-fibrotic therapies, we evaluated the three dimensional ex vivo liver slice (LS) model.
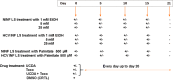
Methods: Fibrotic liver samples (F0 to F4 fibrosis stage according to the METAVIR score) were collected from patients after liver resection. Human liver slices (HLS) were cultivated for up to 21 days. Hepatitis C virus (HCV) infection, alcohol (ethanol stimulation) and steatosis (palmitate stimulation) were examined in fibrotic (F2 to F4) liver slices infected (or not) with HCV. F0-F1 HLS were used as controls. At day 0, either ursodeoxycholic acid (choleretic and hepatoprotective properties) and/or α-tocopherol (antioxidant properties) were added to standard of care on HLS and fibrotic liver slices, infected (or not) with HCV. Expression of the biomarkers of fibrosis and the triglyceride production were checked by quantitative reverse transcription polymerase chain reaction and/or enzyme-linked immunosorbent assay.
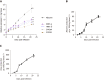
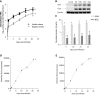
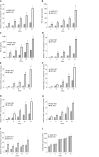
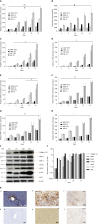
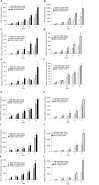
Results: The cultures were viable in vitro for 21 days allowing to study fibrosis inducers and to estimate the effect of anti-fibrotic drugs. Expression of the biomarkers of fibrosis and the progression to steatosis (estimated by triglycerides production) was increased with the addition of HCV and /or ethanol or palmitate. From day 15 of the follow-up studies, a significant decrease of both transforming growth factor β-1 and Procol1A1 expression and triglycerides production was observed when a combined anti-fibrotic treatment was applied on HCV infected F2-F4 LS cultures.
Conclusion: These results show that the human three dimensional ex vivo model effectively reflects the in vivo processes in damaged human liver (viral, alcoholic, nonalcoholic steatohepatitis liver diseases) and provides the proof of concept that the LS examined model permits a rapid evaluation of new anti-fibrotic therapies when used alone or in combination.
Keywords: Alcoholic liver disease; Drugs; Ex vivo model; Hepatitis C virus; Human liver fibrosis; Nonalcoholic steatohepatitis.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: No conflict of interest to declare indicated in the manuscript.
Figures







References
-
- Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. - PubMed
-
- Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JM, Hansen NU, Bay-Jensen AC, Bager CL, Krag A, Blanchard A, Krarup H, Leeming DJ, Schuppan D. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807–G830. - PMC - PubMed
-
- Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P IT-LIVER Consortium. TGF-β signalling and liver disease. FEBS J. 2016;283:2219–2232. - PubMed
-
- van Agthoven M, Metselaar HJ, Tilanus HW, de Man RA, IJzermans JN, Martin van Ineveld BM. A comparison of the costs and effects of liver transplantation for acute and for chronic liver failure. Transpl Int. 2001;14:87–94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

