An implanted device enables in vivo monitoring of extracellular vesicle-mediated spread of pro-inflammatory mast cell response in mice
- PMID: 33708356
- PMCID: PMC7890545
- DOI: 10.1002/jev2.12023
An implanted device enables in vivo monitoring of extracellular vesicle-mediated spread of pro-inflammatory mast cell response in mice
Abstract
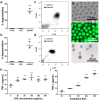
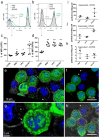
Mast cells have been shown to release extracellular vesicles (EVs) in vitro. However, EV-mediated mast cell communication in vivo remains unexplored. Primary mast cells from GFP-transgenic and wild type mice, were grown in the presence or absence of lipopolysaccharide (LPS), and the secreted EVs were separated from the conditioned media. Mast cell-derived EVs were next cultured with LPS-naïve mast cells, and the induction of TNF-α expression was monitored. In addition, primary mast cells were seeded in diffusion chambers that were implanted into the peritoneal cavities of mice. Diffusion chambers enabled the release of GFP+ mast cell-derived EVs in vivo into the peritoneal cavity. Peritoneal lavage cells were assessed for the uptake of GFP+ EVs and for TNF-α production. In vitro, LPS-stimulated mast cell-derived EVs were efficiently taken up by non-stimulated mast cells, and induced TNF-α expression in a TLR4, JNK and P38 MAPK dependent manner. In vivo, using implanted diffusion chambers, we confirmed the release and transmission of mast cell-derived EVs to other mast cells with subsequent induction of TNF-α expression. These data show an EV-mediated spreading of pro-inflammatory response between mast cells, and provide the first in vivo evidence for the biological role of mast cell-derived EVs.
Keywords: LPS; TNF‐α; extracellular vesicles; in vitro; in vivo; mast cell.
© 2020 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors do not have financial interest to declare.
Figures





References
-
- Al‐Nedawi, K. , Szemraj, J. , & Cierniewski, C. S. (2005). Mast cell‐derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1, Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 1744–1749. - PubMed
-
- Avila, M. , & Gonzalez‐Espinosa, C. (2011). Signaling through Toll‐like receptor 4 and mast cell‐dependent innate immunity responses, Iubmb Life, 63(10), 873–880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

