Lysine methyltransferase G9a is an important modulator of trained immunity
- PMID: 33708384
- PMCID: PMC7890679
- DOI: 10.1002/cti2.1253
Lysine methyltransferase G9a is an important modulator of trained immunity
Abstract
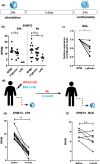
Objectives: Histone methyltransferase G9a, also known as Euchromatic Histone Lysine Methyltransferase 2 (EHMT2), mediates H3K9 methylation which is associated with transcriptional repression. It possesses immunomodulatory effects and is overexpressed in multiple types of cancer. In this study, we investigated the role of G9a in the induction of trained immunity, a de facto innate immune memory, and its effects in non-muscle-invasive bladder cancer (NMIBC) patients treated with intravesical Bacillus Calmette-Guérin (BCG).
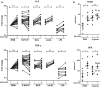
Methods: EHMT2 expression was assessed upon induction of trained immunity by RNA sequencing and Western blotting. G9a inhibitor BIX-01294 was used to investigate the effect on trained immunity responses in vitro. Subsequent cytokine production was measured by ELISA, epigenetic modifications were measured by ChIP-qPCR, Seahorse technology was used to measure metabolic changes, and a luminescence assay was used to measure ROS release. RNA sequencing was performed on BIX-01294-treated monocytes ex vivo.
Results: The expression of EHMT2 mRNA and protein decreased in monocytes during induction of trained immunity. G9a inhibition by BIX-01294 induced trained immunity and amplified trained immunity responses evoked by various microbial ligands in vitro. This was accompanied by decreased H3K9me2 at the promoters of pro-inflammatory genes. G9a inhibition was also associated with amplified ex vivo trained immunity responses in circulating monocytes of NMIBC patients. Additionally, altered RNA expression of inflammatory genes in monocytes of NMIBC patients was observed upon ex vivo G9a inhibition. Furthermore, intravesical BCG therapy decreased H3K9me2 at the promoter of pro-inflammatory genes.
Conclusion: Inhibition of G9a is important in the induction of trained immunity, and G9a may represent a novel therapeutic target in NMIBC patients.
Keywords: BCG; EHMT2; G9a; inflammation; non‐muscle‐invasive bladder cancer; trained immunity.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology Inc.
Conflict of interest statement
MGN and LABJ are scientific founders of Trained Therapeutics Discovery.
Figures




References
-
- Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011; 9: 355–361. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
