Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: rationale and design of the randomized, placebo-controlled HUYGENS study
- PMID: 33708484
- PMCID: PMC7944215
- DOI: 10.21037/cdt-20-684
Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: rationale and design of the randomized, placebo-controlled HUYGENS study
Abstract
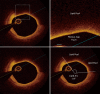
Background: Technological advances in arterial wall imaging permit the opportunity to visualize coronary atherosclerotic plaque with sufficient resolution to characterize both its burden and compositional phenotype. These modalities have been used extensively in clinical trials to evaluate the impact of lipid lowering therapies on serial changes in disease burden. While the findings have unequivocally established that these interventions have the capacity to either slow disease progression or promote plaque regression, depending on the degree of lipid lowering achieved, their impact on plaque phenotype is less certain. More recently optical coherence tomography (OCT) has been employed with a number of studies demonstrating favorable effects on both fibrous cap thickness (FCT) and the size of lipid pools within plaque in response to statin treatment.
Methods: The phase 3, multi-center, double-blind HUYGENS study will assess the impact of incremental lipid lowering with the proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitor, evolocumab, on plaque features using serial OCT imaging, in statin-treated patients following an acute coronary syndrome (ACS). Subjects with non-ST-elevation ACS (n=150) will be randomized 1:1 into two groups to receive monthly injections of evolocumab 420 mg or placebo.
Results: The primary endpoint is the effect of evolocumab on coronary atherosclerotic plaques will be assessed by OCT at baseline and at week 50.
Conclusions: The HUYGENS study will determine whether intensified lipid lowering therapy with evolocumab in addition to maximally tolerated statin therapy will have incremental benefits on high-risk features of coronary artery plaques.
Trial registration: This study was registered on Clinicaltrials.gov (NCT03570697).
Keywords: Imaging; clinical trials; lipids; proprotein convertase subtilisin kexin type 9 (PCSK9); risk factors.
2021 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-684). SJN is a recipient of a Principal Research Fellowship from the National Health and Medical Research Council of Australia and has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx and Sanofi-Regeneron and is a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron and Novo Nordisk. SN reports that the Cleveland Clinic Center for Clinical Research has received funding to perform clinical trials from AbbVie, AstraZeneca, Amgen, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Pfizer, The Medicines Company, Silence Therapeutics, Takeda, and Orexigen. SN is involved in these clinical trials but receives no personal remuneration for his participation. SN consults for many pharmaceutical companies but requires them to donate all honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction. FP received consulting fees from Amgen and Abbott Vascular. SW reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, Sinomed. YK has received research support from Kowa, and speaker honoraria from Abbott Vascular, Amgen, CSL Behring, Daiichi Sankyo, Kowa, Nipro, and Takeda. RP has received lecture fees from Amgen and Sanofi, served as a consultant for Cerenis and on advisory boards for Centerline Biomedical and Medtronic, and holds minor equity in Centerline Biomedical. TH, HK, and JL are employees of Amgen Inc. and hold Amgen stock/stock options. RS is an employee of Epirium Bio, Inc, a former employee of Amgen and identified as an inventor on at least one pending patent application relating to evolocumab. PJP is a recipient of a L2 Future Leader Fellowship from the National Heart Foundation of Australia (FLF102056) and a L2 Career Development Fellowship from the National Health and Medical Research Council of Australia (CDF1161506) and has received research support from Abbott Vascular, consulting fees from Amgen and Esperion and speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Merck Schering-Plough and Pfizer. The other authors have no conflicts of interest to declare.
Figures

References
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082-143. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
