Case Report: Use of Anakinra in Multisystem Inflammatory Syndrome During COVID-19 Pandemic
- PMID: 33708752
- PMCID: PMC7940350
- DOI: 10.3389/fped.2020.624248
Case Report: Use of Anakinra in Multisystem Inflammatory Syndrome During COVID-19 Pandemic
Abstract
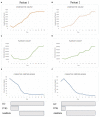
During COVID-19 outbreak, a large number of children with severe inflammatory disease has been reported. This condition, named Pediatric Multi-inflammatory Syndrome temporally associated with COVID-19 (PIMS-TS) or Multisystem Inflammatory Syndrome associated with Coronavirus Disease 2019 (MIS-C), shares some clinical features with Kawasaki disease and is frequently complicated by myocarditis or shock. It has been suggested that MIS-C belongs to the group of cytokine storm syndromes triggered by SARS-CoV-2 infection. So far, intravenous immunoglobulin (IVIG) and systemic glucocorticoids are the most common therapeutic approaches reported in this group of patients. However, the use of anakinra in patients with severe forms of COVID-19 is showing promising results. Here we reported two patients with multisystem inflammatory syndrome complicated with shock. Both the patients presented a poor response to IVIG and systemic glucocorticoids and received anakinra. Treatment with IL-1 receptor antagonist showed a rapid improvement of clinical conditions and biochemical analysis in both patients and demonstrated a good safety profile. Thus, we look forward for future controlled clinical trials with the aim to demonstrate the effectiveness of anakinra in patients with MIS-C and established precise criteria for its use.
Keywords: COVID-19; IL-1; Kawasaki; MISC-C; PIMS-TS; SARS-CoV-2; anakinra.
Copyright © 2021 Della Paolera, Valencic, Piscianz, Moressa, Tommasini, Sagredini, Kiren, Comar and Taddio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous