Model-Informed Precision Dosing of Antibiotics in Pediatric Patients: A Narrative Review
- PMID: 33708753
- PMCID: PMC7940353
- DOI: 10.3389/fped.2021.624639
Model-Informed Precision Dosing of Antibiotics in Pediatric Patients: A Narrative Review
Abstract
Optimal pharmacotherapy in pediatric patients with suspected infections requires understanding and integration of relevant data on the antibiotic, bacterial pathogen, and patient characteristics. Because of age-related physiological maturation and non-maturational covariates (e.g., disease state, inflammation, organ failure, co-morbidity, co-medication and extracorporeal systems), antibiotic pharmacokinetics is highly variable in pediatric patients and difficult to predict without using population pharmacokinetics models. The intra- and inter-individual variability can result in under- or overexposure in a significant proportion of patients. Therapeutic drug monitoring typically covers assessment of pharmacokinetics and pharmacodynamics, and concurrent dose adaptation after initial standard dosing and drug concentration analysis. Model-informed precision dosing (MIPD) captures drug, disease, and patient characteristics in modeling approaches and can be used to perform Bayesian forecasting and dose optimization. Incorporating MIPD in the electronic patient record system brings pharmacometrics to the bedside of the patient, with the aim of a consisted and optimal drug exposure. In this narrative review, we evaluated studies assessing optimization of antibiotic pharmacotherapy using MIPD in pediatric populations. Four eligible studies involving amikacin and vancomycin were identified from 418 records. Key articles, independent of year of publication, were also selected to highlight important attributes of MIPD. Although very little research has been conducted until this moment, the available data on vancomycin indicate that MIPD is superior compared to conventional dosing strategies with respect to target attainment. The utility of MIPD in pediatrics needs to be further confirmed in frequently used antibiotic classes, particularly aminoglycosides and beta-lactams.
Keywords: Bayesian; antibiotics; model-informed precision dosing; neonates; pediatric; population PK models; therapeutic drug monitoring.
Copyright © 2021 Abdulla, Edwina, Flint, Allegaert, Wildschut, Koch and de Hoog.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
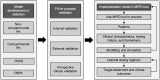
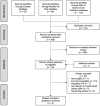
References
-
- Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H. The antibiotic resistance and prescribing in european children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. (2013) 32:e242–53. 10.1097/INF.0b013e318286c612 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

