Omics-Driven Biotechnology for Industrial Applications
- PMID: 33708762
- PMCID: PMC7940536
- DOI: 10.3389/fbioe.2021.613307
Omics-Driven Biotechnology for Industrial Applications
Abstract
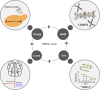
Biomanufacturing is a key component of biotechnology that uses biological systems to produce bioproducts of commercial relevance, which are of great interest to the energy, material, pharmaceutical, food, and agriculture industries. Biotechnology-based approaches, such as synthetic biology and metabolic engineering are heavily reliant on "omics" driven systems biology to characterize and understand metabolic networks. Knowledge gained from systems biology experiments aid the development of synthetic biology tools and the advancement of metabolic engineering studies toward establishing robust industrial biomanufacturing platforms. In this review, we discuss recent advances in "omics" technologies, compare the pros and cons of the different "omics" technologies, and discuss the necessary requirements for carrying out multi-omics experiments. We highlight the influence of "omics" technologies on the production of biofuels and bioproducts by metabolic engineering. Finally, we discuss the application of "omics" technologies to agricultural and food biotechnology, and review the impact of "omics" on current COVID-19 research.
Keywords: biotechnology; genomics; metabolic engineering; metabolomics; multi-omics; proteomics; systems biology; transcriptomics.
Copyright © 2021 Amer and Baidoo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abid F., Zahid M. A., Abedin Z. U., Nizami S. B., Abid M. J., Kazmi S. Z. H., et al. . (2018). Omics Approaches in Marine Biotechnology: The Treasure of Ocean for Human Betterments. London: Elsevier Inc.
-
- Ahmed Z., Zeeshan S., Foran D. J., Kleinman L. C., Wondisford F. E., Dong X. Q. (2020). Integrative clinical, genomics and metabolomics data analysis for mainstream precision medicine to investigate COVID-19. BMJ Innov. 7, 1–5. 10.1136/bmjinnov-2020-000444 - DOI
-
- Alfaro M. P., Sepulveda J. L., Lyon E. (2019). Molecular testing for targeted therapies and pharmacogenomics, in Accurate Results in the Clinical Laboratory, eds Dasgupta A., Sepulveda J. L. (Amsterdam: Elsevier; ), 349–363. 10.1016/b978-0-12-813776-5.00022-4 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

