Prospective Comparison of Saliva and Nasopharyngeal Swab Sampling for Mass Screening for COVID-19
- PMID: 33708779
- PMCID: PMC7940378
- DOI: 10.3389/fmed.2021.621160
Prospective Comparison of Saliva and Nasopharyngeal Swab Sampling for Mass Screening for COVID-19
Abstract
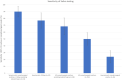
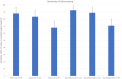
Current testing for COVID-19 relies on reverse-transcriptase polymerase chain reaction from a nasopharyngeal swab specimen. Saliva samples have advantages regarding ease and painlessness of collection, which does not require trained staff and may allow self-sampling. We enrolled 776 persons at various field-testing sites and collected nasopharyngeal and pooled saliva samples. One hundred sixty two had a positive COVID-19 RT-PCR, 61% were mildly symptomatic and 39% asymptomatic. The sensitivity of RT-PCR on saliva samples vs. nasopharygeal swabs varied depending on the patient groups considered or on Ct thresholds. There were 10 (6.2%) patients with a positive saliva sample and a negative nasopharyngeal swab, all of whom had Ct values <25 for three genes. For symptomatic patients for whom the interval between symptoms onset and sampling was <10 days sensitivity was 77% but when excluding persons with isolated N gene positivity (54/162), sensitivity was 90%. In asymptomatic patients, the sensitivity was only 24%. When we looked at patients with Cts <30, sensitivity was 83 or 88.9% when considering two genes. The relatively good performance for patients with low Cts suggests that Saliva testing could be a useful and acceptable tool to identify infectious persons in mass screening contexts, a strategically important task for contact tracing and isolation in the community.
Keywords: COVID-19; PCR; nasopharyngeal; saliva; sensitivity.
Copyright © 2021 Nacher, Mergeay-Fabre, Blanchet, Benoit, Pozl, Mesphoule, Sainte-Rose, Vialette, Toulet, Moua, Saout, Simon, Guidarelli, Galindo, Biche, Faurous, Chaizemartin, Fahrasmane, Rochemont, Vignier, Vabret and Demar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

