An analytical study of drug utilization, disease progression, and adverse events among 165 COVID-19 patients
- PMID: 33708933
- PMCID: PMC7944318
- DOI: 10.21037/atm-20-4960
An analytical study of drug utilization, disease progression, and adverse events among 165 COVID-19 patients
Abstract
Background: The coronavirus disease 2019 (COVID-19) epidemic has lasted for nearly 4 months by this study was conducted. We aimed to describe drug utilization, disease progression, and adverse drug events of COVID-19.
Methods: A retrospective, single-center case series study enrolled 165 consecutive hospitalized COVID-19 patients who were followed up until March 25, 2020, from a designated hospital in Wuhan. Patients were grouped by a baseline degree of severity: non-severe and severe. An analytical study of drug utilization, disease progression, and adverse events (AEs) of COVID-19 was conducted.
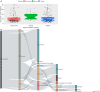
Results: Of the 165 COVID-19 cases, antivirals, antibacterials, glucocorticoids, and traditional Chinese medicine (TCM) were administered to 92.7%, 98.8%, 68.5%, and 55.2% of patients, respectively. The total kinds of drugs administered to the severe subgroup [26, interquartile range (IQR) 18-39] were 11 more than the non-severe subgroup (15, IQR 10-24), regardless of comorbidities. The 2 most common combinations of medications in the 165 cases were 'antiviral therapy + glucocorticoids + TCM' (81, 49.1%) and 'antiviral therapy + glucocorticoids' (23, 13.9%). Compared with non-severe cases, severe cases received more glucocorticoids (88.5% vs. 66.2%, P=0.02), but less TCM (50.0% vs. 63.3%, P=0.20), and suffered a higher percentage of death (34.6% vs. 7.2%, P=0.001). At the end of the follow-up, 130 (78.8%) patients had been discharged, and 24 (14.5%) died. There were 13 patients (7.9%) who had elevated liver enzymes, and 49 patients (29.7%) presented with worsening kidney function during the follow-up.
Conclusions: Of the 165 COVID-19 patients, the fatality rate remained high (14.5%). Drug utilization for COVID-19 was diverse and generally complied with the existing guidelines. Combination regimens containing antiviral drugs might be beneficial to assist COVID-19 recovery. Additionally, liver and kidney AEs should not be ignored.
Keywords: Novel coronavirus disease (COVID-19); adverse events (AEs); disease progression; drug utilization; fatality.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4960). Dr. SZ reports grants from National Key Technology R&D Program of China, grants from National Natural Science Foundation of China, grants from Special Project for Major Infectious Diseases of Peking University Health Science Center, during the conduct of the study. Dr. YC reports grants from Fundamental Research Funds for the Central Universities, during the conduct of the study. The other authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
