Container Closure and Delivery Considerations for Intravitreal Drug Administration
- PMID: 33709236
- PMCID: PMC7952281
- DOI: 10.1208/s12249-021-01949-4
Container Closure and Delivery Considerations for Intravitreal Drug Administration
Abstract
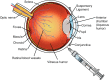
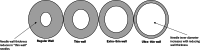
Intravitreal (IVT) administration of therapeutics is the standard of care for treatment of back-of-eye disorders. Although a common procedure performed by retinal specialists, IVT administration is associated with unique challenges related to drug product, device and the procedure, which may result in adverse events. Container closure configuration plays a crucial role in maintaining product stability, safety, and efficacy for the intended shelf-life. Careful design of primary container configuration is also important to accurately deliver small volumes (10-100 μL). Over- or under-dosing may lead to undesired adverse events or lack of efficacy resulting in unpredictable and variable clinical responses. IVT drug products have been traditionally presented in glass vials. However, pre-filled syringes offer a more convenient administration option by reducing the number of steps required for dose preparation there by potentially reducing the time demand on the healthcare providers. In addition to primary container selection, product development studies should focus on, among other things, primary container component characterization, material compatibility with the formulation, formulation stability, fill volume determination, extractables/leachables, and terminal sterilization. Ancillary components such as disposable syringes and needles must be carefully selected, and a detailed administration procedure that includes dosing instructions is required to ensure successful administration of the product. Despite significant efforts in improving the drug product and administration procedures, ocular safety concerns such as endophthalmitis, increased intraocular pressure, and presence of silicone floaters have been reported. A systematic review of available literature on container closure and devices for IVT administration can help guide successful product development.
Keywords: Formulation; Needle; Ocular; Sterilization; Syringe.
Figures


References
-
- Melo GB, Cruz N, Emerson GG, Rezende FA, Meyer CH, Uchiyama S, et al. Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Prog Retin Eye Res. 2020;100862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

