Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution
- PMID: 33709682
- PMCID: PMC8892434
- DOI: 10.1021/acsami.1c02338
Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution
Abstract
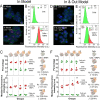
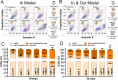
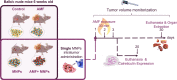
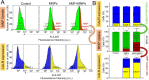
Magnetic hyperthermia (MH) was used to treat a murine model of pancreatic cancer. This type of cancer is generally characterized by the presence of dense stroma that acts as a barrier for chemotherapeutic treatments. Several alternating magnetic field (AMF) conditions were evaluated using three-dimensional (3D) cell culture models loaded with magnetic nanoparticles (MNPs) to determine which conditions were producing a strong effect on the cell viability. Once the optimal AMF conditions were selected, in vivo experiments were carried out using similar frequency and field amplitude parameters. A marker of the immune response activation, calreticulin (CALR), was evaluated in cells from a xenograft tumor model after the MH treatment. Moreover, the distribution of nanoparticles within the tumor tissue was assessed by histological analysis of tumor sections, observing that the exposure to the alternating magnetic field resulted in the migration of particles toward the inner parts of the tumor. Finally, a relationship between an inadequate body biodistribution of the particles after their intratumoral injection and a significant decrease in the effectiveness of the MH treatment was found. Animals in which most of the particles remained in the tumor area after injection showed higher reductions in the tumor volume growth in comparison with those animals in which part of the particles were found also in the liver and spleen. Therefore, our results point out several factors that should be considered to improve the treatment effectiveness of pancreatic cancer by magnetic hyperthermia.
Keywords: biodistribution; immunological effect; intratumor administration; iron oxide magnetic nanoparticles; magnetic hyperthermia; pancreatic cancer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

