Emulsion-free chitosan-genipin microgels for growth plate cartilage regeneration
- PMID: 33709832
- PMCID: PMC8319035
- DOI: 10.1177/0885328221999894
Emulsion-free chitosan-genipin microgels for growth plate cartilage regeneration
Abstract
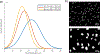
The growth plate is a cartilage tissue near the ends of children's long bones and is responsible for bone growth. Injury to the growth plate can result in the formation of a 'bony bar' which can span the growth plate and result in bone growth abnormalities in children. Biomaterials such as chitosan microgels could be a potential treatment for growth plate injuries due to their chondrogenic properties, which can be enhanced through loading with biologics. They are commonly fabricated via an emulsion method, which involves solvent rinses that are cytotoxic. Here, we present a high throughput, non-cytotoxic, non-emulsion-based method to fabricate chitosan-genipin microgels. Chitosan was crosslinked with genipin to form a hydrogel network, and then pressed through a syringe filter using mesh with various pore sizes to produce a range of microgel particle sizes. The microgels were then loaded with chemokines and growth factors and their release was studied in vitro. To assess the applicability of the microgels for growth plate cartilage regeneration, they were injected into a rat growth plate injury. They led to increased cartilage repair tissue and were fully degraded by 28 days in vivo. This work demonstrates that chitosan microgels can be fabricated without solvent rinses and demonstrates their potential for the treatment of growth plate injuries.
Keywords: Chitosan; cartilage regeneration; growth plate; injectable biomaterials; microgels.
Figures
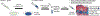
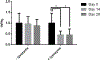

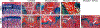
References
-
- Karnchanajindanun J, Srisa-ard M and Baimark Y. Genipin-cross-linked chitosan microspheres prepared by a water-in-oil emulsion solvent diffusion method for protein delivery. Carbohydrate Polymers 2011; 85: 674–680. DOI: 10.1016/j.carbpol.2011.03.035. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

