Conducting Real-world Evidence Studies on the Clinical Outcomes of Diabetes Treatments
- PMID: 33710268
- PMCID: PMC8476933
- DOI: 10.1210/endrev/bnab007
Conducting Real-world Evidence Studies on the Clinical Outcomes of Diabetes Treatments
Erratum in
-
Erratum to: "Conducting Real-world Evidence Studies on the Clinical Outcomes of Diabetes Treatments".Endocr Rev. 2021 Nov 16;42(6):873. doi: 10.1210/endrev/bnab032. Endocr Rev. 2021. PMID: 34590676 Free PMC article. No abstract available.
Abstract
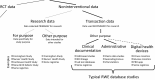
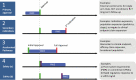
Real-world evidence (RWE), the understanding of treatment effectiveness in clinical practice generated from longitudinal patient-level data from the routine operation of the healthcare system, is thought to complement evidence on the efficacy of medications from randomized controlled trials (RCTs). RWE studies follow a structured approach. (1) A design layer decides on the study design, which is driven by the study question and refined by a medically informed target population, patient-informed outcomes, and biologically informed effect windows. Imagining the randomized trial we would ideally perform before designing an RWE study in its likeness reduces bias; the new-user active comparator cohort design has proven useful in many RWE studies of diabetes treatments. (2) A measurement layer transforms the longitudinal patient-level data stream into variables that identify the study population, the pre-exposure patient characteristics, the treatment, and the treatment-emergent outcomes. Working with secondary data increases the measurement complexity compared to primary data collection that we find in most RCTs. (3) An analysis layer focuses on the causal treatment effect estimation. Propensity score analyses have gained in popularity to minimize confounding in healthcare database analyses. Well-understood investigator errors, like immortal time bias, adjustment for causal intermediates, or reverse causation, should be avoided. To increase reproducibility of RWE findings, studies require full implementation transparency. This article integrates state-of-the-art knowledge on how to conduct and review RWE studies on diabetes treatments to maximize study validity and ultimately increased confidence in RWE-based decision making.
Keywords: Diabetes; bias; causal treatment effects; confounding; healthcare databases; measurement; pharmacoepidemiology; real-world evidence; regulatory decisions.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures











References
-
- Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. - PubMed
-
- Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

