Mesoscopic fluorescence lifetime imaging: Fundamental principles, clinical applications and future directions
- PMID: 33710785
- PMCID: PMC8579869
- DOI: 10.1002/jbio.202000472
Mesoscopic fluorescence lifetime imaging: Fundamental principles, clinical applications and future directions
Abstract
Fluorescence lifetime imaging (FLIm) is an optical spectroscopic imaging technique capable of real-time assessments of tissue properties in clinical settings. Label-free FLIm is sensitive to changes in tissue structure and biochemistry resulting from pathological conditions, thus providing optical contrast to identify and monitor the progression of disease. Technical and methodological advances over the last two decades have enabled the development of FLIm instrumentation for real-time, in situ, mesoscopic imaging compatible with standard clinical workflows. Herein, we review the fundamental working principles of mesoscopic FLIm, discuss the technical characteristics of current clinical FLIm instrumentation, highlight the most commonly used analytical methods to interpret fluorescence lifetime data and discuss the recent applications of FLIm in surgical oncology and cardiovascular diagnostics. Finally, we conclude with an outlook on the future directions of clinical FLIm.
Keywords: FLIm; cardiovascular imaging; fluorescence lifetime imaging; image-guided surgery; intraoperative tumor delineation.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflict of interests.
Figures



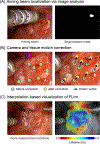

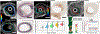
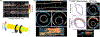
Similar articles
-
Applications of machine learning in time-domain fluorescence lifetime imaging: a review.Methods Appl Fluoresc. 2024 Feb 8;12(2):022001. doi: 10.1088/2050-6120/ad12f7. Methods Appl Fluoresc. 2024. PMID: 38055998 Free PMC article. Review.
-
Recent innovations in fluorescence lifetime imaging microscopy for biology and medicine.J Biomed Opt. 2021 Jul;26(7):070603. doi: 10.1117/1.JBO.26.7.070603. J Biomed Opt. 2021. PMID: 34247457 Free PMC article.
-
Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications.J Biomed Opt. 2020 May;25(7):1-43. doi: 10.1117/1.JBO.25.7.071203. J Biomed Opt. 2020. PMID: 32406215 Free PMC article. Review.
-
Quantitative Imaging of Genetically Encoded Fluorescence Lifetime Biosensors.Biosensors (Basel). 2023 Oct 19;13(10):939. doi: 10.3390/bios13100939. Biosensors (Basel). 2023. PMID: 37887132 Free PMC article. Review.
-
Fluorescence lifetime imaging in drug delivery research.Adv Drug Deliv Rev. 2025 Mar;218:115521. doi: 10.1016/j.addr.2025.115521. Epub 2025 Jan 21. Adv Drug Deliv Rev. 2025. PMID: 39848547 Review.
Cited by
-
Applications of machine learning in time-domain fluorescence lifetime imaging: a review.Methods Appl Fluoresc. 2024 Feb 8;12(2):022001. doi: 10.1088/2050-6120/ad12f7. Methods Appl Fluoresc. 2024. PMID: 38055998 Free PMC article. Review.
-
Intraoperative molecular imaging: 3rd biennial clinical trials update.J Biomed Opt. 2023 May;28(5):050901. doi: 10.1117/1.JBO.28.5.050901. Epub 2023 May 13. J Biomed Opt. 2023. PMID: 37193364 Free PMC article. Review.
-
Intraoperative detection of IDH-mutant glioma using fluorescence lifetime imaging.J Biophotonics. 2023 Apr;16(4):e202200291. doi: 10.1002/jbio.202200291. Epub 2022 Dec 23. J Biophotonics. 2023. PMID: 36510639 Free PMC article.
-
AlliGator: Open Source Fluorescence Lifetime Imaging Analysis in G.bioRxiv [Preprint]. 2025 Jun 18:2025.05.22.655640. doi: 10.1101/2025.05.22.655640. bioRxiv. 2025. PMID: 40501943 Free PMC article. Preprint.
-
Defining the Zone of Acute Peripheral Nerve Injury Using Fluorescence Lifetime Imaging in a Crush Injury Sheep Model.J Hand Surg Am. 2025 Jan 4:S0363-5023(24)00605-1. doi: 10.1016/j.jhsa.2024.11.020. Online ahead of print. J Hand Surg Am. 2025. PMID: 39755964 Free PMC article.
References
-
- Zaffino P, Moccia S, De Momi E, Spadea MF, Ann. Biomed. Eng 2020, 48, 2171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

