Role of perivascular nerve and sensory neurotransmitter dysfunction in inflammatory bowel disease
- PMID: 33710922
- PMCID: PMC8163646
- DOI: 10.1152/ajpheart.00037.2021
Role of perivascular nerve and sensory neurotransmitter dysfunction in inflammatory bowel disease
Abstract
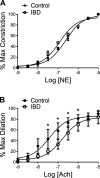
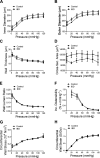
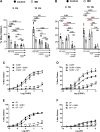
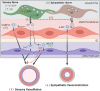
Inflammatory bowel disease (IBD) is associated with both impaired intestinal blood flow and increased risk of cardiovascular disease, but the functional role of perivascular nerves that control vasomotor function of mesenteric arteries (MAs) perfusing the intestine during IBD is unknown. Because perivascular sensory nerves and their transmitters calcitonin gene-related peptide (CGRP) and substance P (SP) are important mediators of both vasodilation and inflammatory responses, our objective was to identify IBD-related deficits in perivascular sensory nerve function and vascular neurotransmitter signaling. In MAs from an interleukin-10 knockout (IL-10-/-) mouse model, IBD significantly impairs electrical field stimulation (EFS)-mediated sensory vasodilation and inhibition of sympathetic vasoconstriction, despite decreased sympathetic nerve density and vasoconstriction. The MA content and EFS-mediated release of both CGRP and SP are decreased with IBD, but IBD has unique effects on each transmitter. CGRP nerve density, receptor expression, hyperpolarization, and vasodilation are preserved with IBD. In contrast, SP nerve density and receptor expression are increased, and SP hyperpolarization and vasodilation are impaired with IBD. A key finding is that blockade of SP receptors restores EFS-mediated sensory vasodilation and enhanced CGRP-mediated vasodilation in MAs from IBD but not Control mice. Together, these data suggest that an aberrant role for the perivascular sensory neurotransmitter SP and its downstream signaling in MAs underlies vascular dysfunction with IBD. We propose that with IBD, SP signaling impedes CGRP-mediated sensory vasodilation, contributing to impaired blood flow. Thus, substance P and NK1 receptors may represent an important target for treating vascular dysfunction in IBD.NEW & NOTEWORTHY Our study is the first to show that IBD causes profound impairment of sensory vasodilation and inhibition of sympathetic vasoconstriction in mesenteric arteries. This occurs alongside decreased SP-containing nerve density and increased expression of NK1 receptors for SP. In contrast, CGRP dilation, nerve density, and receptor expression are unchanged. Blocking NK1 receptors restores sensory vasodilation in MAs and increases CGRP-mediated vasodilation, indicating that SP interference with CGRP signaling may underlie impaired sensory vasodilation with IBD.
Keywords: adventitia; calcitonin gene-related peptide; inflammation; substance P; sympathetic nerves; vasodilation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age.J Physiol. 2016 Apr 15;594(8):2323-38. doi: 10.1113/JP270710. Epub 2015 Jun 30. J Physiol. 2016. PMID: 26010764 Free PMC article.
-
Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice.J Hypertens. 2006 Dec;24(12):2399-408. doi: 10.1097/01.hjh.0000251900.78051.56. J Hypertens. 2006. PMID: 17082722
-
Characterization of Perivascular Nerve Distribution in Rat Mesenteric Small Arteries.Biol Pharm Bull. 2015;38(11):1757-64. doi: 10.1248/bpb.b15-00461. Biol Pharm Bull. 2015. PMID: 26521827
-
The effects of CGRP in vascular tissue - Classical vasodilation, shadowed effects and systemic dilemmas.Eur J Pharmacol. 2020 Aug 15;881:173205. doi: 10.1016/j.ejphar.2020.173205. Epub 2020 May 19. Eur J Pharmacol. 2020. PMID: 32442540 Review.
-
[Vasodilator nerves].Yakugaku Zasshi. 1994 Oct;114(10):765-74. doi: 10.1248/yakushi1947.114.10_765. Yakugaku Zasshi. 1994. PMID: 7807380 Review. Japanese.
Cited by
-
Neurokinin receptors and their implications in various autoimmune diseases.Curr Res Immunol. 2021 Jul 1;2:66-78. doi: 10.1016/j.crimmu.2021.06.001. eCollection 2021. Curr Res Immunol. 2021. PMID: 35492389 Free PMC article. Review.
-
The metabolites of gut microbiota: their role in ferroptosis in inflammatory bowel disease.Eur J Med Res. 2025 Apr 7;30(1):248. doi: 10.1186/s40001-025-02524-4. Eur J Med Res. 2025. PMID: 40189555 Free PMC article. Review.
-
An American Physiological Society cross-journal Call for Papers on "Inter-Organ Communication in Homeostasis and Disease".Am J Physiol Lung Cell Mol Physiol. 2021 Jul 1;321(1):L42-L49. doi: 10.1152/ajplung.00209.2021. Epub 2021 May 19. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34010064 Free PMC article. No abstract available.
-
Adventitial macrophage accumulation impairs perivascular nerve function in mesenteric arteries with inflammatory bowel disease.Front Physiol. 2023 May 25;14:1198066. doi: 10.3389/fphys.2023.1198066. eCollection 2023. Front Physiol. 2023. PMID: 37342800 Free PMC article.
-
Recovery of Ischemic Limb and Femoral Artery Endothelial Function Are Preserved in Mice with Dextran Sodium Sulfate-Induced Chronic Colitis.Biology (Basel). 2022 Aug 4;11(8):1169. doi: 10.3390/biology11081169. Biology (Basel). 2022. PMID: 36009796 Free PMC article.
References
-
- Papa A, Danese S, Urgesi R, Grillo A, Guglielmo S, Roberto I, Bonizzi M, Guidi L, De Vitis I, Santoliquido A, Fedeli G, Gasbarrini G, Gasbarrini A. Early atherosclerosis in patients with inflammatory bowel disease. Eur Rev Med Pharmacol Sci 10: 7–11, 2006. - PubMed
-
- Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H, Novacek G. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut 53: 542–548, 2004. doi:10.1136/gut.2003.025411. - DOI - PMC - PubMed
-
- Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Lamberts M, Khalid U, Nielsen OH, Torp-Pedersen C, Gislason GH, Hansen PR. Inflammatory bowel disease is associated with an increased risk of hospitalization for heart failure: a Danish nationwide cohort study. Circ Heart Fail 7: 717–722, 2014. doi:10.1161/CIRCHEARTFAILURE.114.001152. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

