Multiplexing physical stimulation on single human induced pluripotent stem cell-derived cardiomyocytes for phenotype modulation
- PMID: 33710972
- PMCID: PMC7610872
- DOI: 10.1088/1758-5090/abce0a
Multiplexing physical stimulation on single human induced pluripotent stem cell-derived cardiomyocytes for phenotype modulation
Abstract
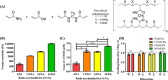
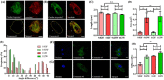
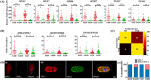
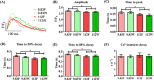
Traditional in vitro bioengineering approaches whereby only individual biophysical cues are manipulated at any one time are highly inefficient, falling short when recapitulating the complexity of the cardiac environment. Multiple biophysical cues are present in the native myocardial niche and are essential during development, as well as in maintenance of adult cardiomyocyte (CM) phenotype in both health and disease. This study establishes a novel biofabrication workflow to study and manipulate hiPSC-CMs and to understand how these cells respond to a multiplexed biophysical environment, namely 3D shape and substrate stiffness, at a single cell level. Silicon masters were fabricated and developed to generate inverse patterns of the desired 3D shapes in bas relief, which then were used to mold the designed microwell arrays into a hydrogel. Polyacrylamide (PAAm) was modified with the incorporation of acrylic acid to provide a carboxylic group conjugation site for adhesion motifs, without compromising capacity to modulate stiffness. In this manner, two individual parameters can be finely tuned independently within the hydrogel: the shape of the 3D microwell and its stiffness. The design allows the platform to isolate single hiPSC-CMs to study solely biophysical cues in the absence of cell-cell physical interaction. Under physiologic-like physical conditions (3D shape resembling that of adult CM and 9.83 kPa substrate stiffness that mimics muscle stiffness), isolated single hiPSC-CMs exhibit increased Cx-43 density, cell membrane stiffness and calcium transient amplitude; co-expression of the subpopulation-related MYL2-MYL7 proteins; and higher anisotropism than cells in pathologic-like conditions (flat surface and 112 kPa substrate stiffness). This demonstrates that supplying a physiologic or pathologic microenvironment to an isolated single hiPSC-CM in the absence of any physical cell-to-cell communication in this biofabricated platform leads to a significantly different set of cellular features, thus presenting a differential phenotype. Importantly, this demonstrates the high plasticity of hiPSC-CMs even in isolation. The ability of multiple biophysical cues to significantly influence isolated single hiPSC-CM phenotype and functionality highlights the importance of fine-tuning such cues for specific applications. This has the potential to produce more fit-for-purpose hiPSC-CMs. Further understanding of human cardiac development is enabled by the robust, versatile and reproducible biofabrication techniques applied here. We envision that this system could be easily applied to other tissues and cell types where the influence of cellular shape and stiffness of the surrounding environment is hypothesized to play an important role in physiology.
Figures

References
-
- Lian X, Zhang J, Azarin S M, Zhu K, Hazeltine L B, Bao X, Hsiao C, Kamp T J, Palecek S P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protocols. 2013;8:162–75. doi: 10.1038/nprot.2012.150. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
