Randomised study: effects of the 5-HT4 receptor agonist felcisetrag vs placebo on gut transit in patients with gastroparesis
- PMID: 33711180
- PMCID: PMC8251541
- DOI: 10.1111/apt.16304
Randomised study: effects of the 5-HT4 receptor agonist felcisetrag vs placebo on gut transit in patients with gastroparesis
Abstract
Background: Gastroparesis is defined by delayed gastric emptying with associated symptoms in the absence of mechanical obstruction.
Aim: To evaluate pharmacokinetics and pharmacodynamics of felcisetrag, a highly selective 5-HT4 receptor agonist, on total gut transit in patients with documented delayed gastric emptying of solids.
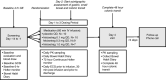
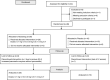
Methods: Single-centre, placebo-controlled study of 36 participants receiving placebo, 0.1mg, 0.3mg or 1.0mg of felcisetrag I.V. infusion, daily, for 3 days. At baseline, each participant completed a 4h, 99m Tc-egg meal (300 kcal, 30% fat) gastric emptying test. Following infusion (Day 2), gastric, small bowel and colonic transit of solids were measured over 48h (same meal plus 111 In-charcoal delivered in methacrylate-coated capsule). Samples were collected for pharmacokinetics. The primary endpoint was gastric emptying T1/2 . Statistical analysis used baseline parameters as covariates (ANCOVA).
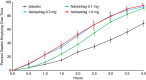
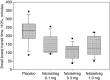
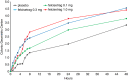
Results: Patients (22 idiopathic, 14 diabetic gastroparesis) were randomised to felcisetrag (0.1 mg, n = 10; 0.3 mg, n = 9; 1.0 mg, n = 7) or placebo (n = 10). Compared to placebo, felcisetrag significantly accelerated gastric emptying T1/2 , colonic filling at 6h, and 10% small bowel transit time (overall P < 0.01; all three doses individually Bonferroni corrected P < 0.05) for all three measurements. Ascending colon emptying (T1/2 ) was significantly accelerated (all doses), and colonic transit at 48 hours was accelerated with 0.1 mg and 0.3 mg felcisetrag compared to placebo. Pharmacokinetic results were dose proportional. Felcisetrag was well tolerated with no clinically significant findings from clinical laboratory, vital signs or ECG.
Conclusion: I.V. felcisetrag significantly accelerated gastric, small bowel and colonic transit in patients with gastroparesis, and should be further evaluated for short-term treatment of gastric and intestinal motility disorders. ClinicalTrials.gov #NCT03281577.
© 2021 Mayo Foundation for Medical and Research. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

Comment in
-
Editorial: felcisetrag-forward movement as a novel prokinetic for gastroparesis.Aliment Pharmacol Ther. 2021 May;53(10):1158-1159. doi: 10.1111/apt.16334. Aliment Pharmacol Ther. 2021. PMID: 33882167 No abstract available.
References
-
- Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nature Reviews Disease Primers. 2018;4:41. - PubMed
-
- Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. - PubMed
-
- Park S‐Y, Acosta A, Camilleri M, et al. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol. 2017;112:1689–1699. - PubMed
-
- Chedid V, Brandler J, Vijayvargiya P, et al. Characterization of upper gastrointestinal symptoms, gastric motor functions, and associations in patients with diabetes at a referral center. Am J Gastroenterol. 2019;114:143–154. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
