Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo
- PMID: 33711211
- PMCID: PMC8045927
- DOI: 10.1111/acel.13338
Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo
Abstract
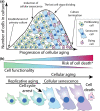
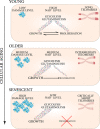
The field of research on cellular senescence experienced a rapid expansion from being primarily focused on in vitro aspects of aging to the vast territories of animal and clinical research. Cellular senescence is defined by a set of markers, many of which are present and accumulate in a gradual manner prior to senescence induction or are found outside of the context of cellular senescence. These markers are now used to measure the impact of cellular senescence on aging and disease as well as outcomes of anti-senescence interventions, many of which are at the stage of clinical trials. It is thus of primary importance to discuss their specificity as well as their role in the establishment of senescence. Here, the presence and role of senescence markers are described in cells prior to cell cycle arrest, especially in the context of replicative aging and in vivo conditions. Specifically, this review article seeks to describe the process of "cellular aging": the progression of internal changes occurring in primary cells leading to the induction of cellular senescence and culminating in cell death. Phenotypic changes associated with aging prior to senescence induction will be characterized, as well as their effect on the induction of cell senescence and the final fate of cells reviewed. Using published datasets on assessments of senescence markers in vivo, it will be described how disparities between quantifications can be explained by the concept of cellular aging. Finally, throughout the article the applicational value of broadening cellular senescence paradigm will be discussed.
Keywords: aging; cellular senescence; evolutionary biology; molecular biology of aging; molecular damage; theories of aging; wound healing.
© 2021 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T.‐W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. 10.1038/ncb2784 - DOI - PMC - PubMed
-
- Ahmed, S. , Passos, J. F. , Birket, M. J. , Beckmann, T. , Brings, S. , Peters, H. , Birch‐Machin, M. A. , von Zglinicki, T. , & Saretzki, G. (2008). Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. Journal of Cell Science, 121(Pt 7), 1046–1053. 10.1242/jcs.019372 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

