Simvastatin inhibits POVPC-mediated induction of endothelial-to-mesenchymal cell transition
- PMID: 33711324
- PMCID: PMC8063863
- DOI: 10.1016/j.jlr.2021.100066
Simvastatin inhibits POVPC-mediated induction of endothelial-to-mesenchymal cell transition
Abstract
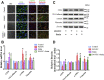
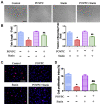
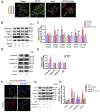
Endothelial-to-mesenchymal transition (EndMT), the process by which an endothelial cell (EC) undergoes a series of molecular events that result in a mesenchymal cell phenotype, plays an important role in atherosclerosis. 1-Palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), derived from the oxidation of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine, is a proinflammatory lipid found in atherosclerotic lesions. Whether POVPC promotes EndMT and how simvastatin influences POVPC-mediated EndMT remains unclear. Here, we treated human umbilical vein ECs with POVPC, simvastatin, or both, and determined their effect on EC viability, morphology, tube formation, proliferation, and generation of NO and superoxide anion (O2•-). Expression of specific endothelial and mesenchymal markers was detected by immunofluorescence and immunoblotting. POVPC did not affect EC viability but altered cellular morphology from cobblestone-like ECs to a spindle-like mesenchymal cell morphology. POVPC increased O2- generation and expression of alpha-smooth muscle actin, vimentin, Snail-1, Twist-1, transforming growth factor-beta (TGF-β), TGF-β receptor II, p-Smad2/3, and Smad2/3. POVPC also decreased NO production and expression of CD31 and endothelial NO synthase. Simvastatin inhibited POVPC-mediated effects on cellular morphology, production of O2•- and NO, and expression of specific endothelial and mesenchymal markers. These data demonstrate that POVPC induces EndMT by increasing oxidative stress, which stimulates TGF-β/Smad signaling, leading to Snail-1 and Twist-1 activation. Simvastatin inhibited POVPC-induced EndMT by decreasing oxidative stress, suppressing TGF-β/Smad signaling, and inactivating Snail-1 and Twist-1. Our findings reveal a novel mechanism of atherosclerosis that can be inhibited by simvastatin.
Keywords: CVD; LDL; NO; atherosclerosis; cell biology; endothelial cells; oxidized lipids; signal transduction; superoxide anion; vascular biology.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Nazarian-Samani Z., Sewell R.D.E., Rafieian-Kopaei M. Inflammasome signaling and other factors implicated in atherosclerosis development and progression. Curr. Pharm. Des. 2020;26:2583–2590. - PubMed
-
- Ranchoux B., Antigny F., Rucker-Martin C., Hautefort A., Pechoux C., Bogaard H.J., Dorfmuller P., Remy S., Lecerf F., Plante S., Chat S., Fadel E., Houssaini A., Anegon I., Adnot S. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

