Handling and accuracy of four rapid antigen tests for the diagnosis of SARS-CoV-2 compared to RT-qPCR
- PMID: 33711691
- PMCID: PMC7927591
- DOI: 10.1016/j.jcv.2021.104782
Handling and accuracy of four rapid antigen tests for the diagnosis of SARS-CoV-2 compared to RT-qPCR
Abstract
Background: SARS-CoV-2 molecular diagnostics is facing material shortages and long turnaround times due to exponential increase of testing demand.
Objective: We evaluated the analytic performance and handling of four rapid Antigen Point of Care Tests (AgPOCTs) I-IV (Distributors: (I) Roche, (II) Abbott, (III) MEDsan and (IV) Siemens).
Methods: 100 RT-PCR negative and 84 RT-PCR positive oropharyngeal swabs were prospectively collected and used to determine performance and accuracy of these AgPOCTs. Handling was evaluated by 10 healthcare workers/users through a questionnaire.
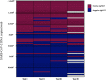
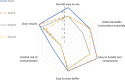
Results: The median duration from symptom onset to sampling was 6 days (IQR 2-12 days). The overall respective sensitivity were 49.4 % (CI95 %: 38.9-59.9), 44.6 % (CI95 %: 34.3-55.3), 45.8 % (CI95 %: 35.5-56.5) and 54.9 % (CI95 %: 43.4-65.9) for tests I, II, III and IV, respectively. In the high viral load subgroup (containing >106 copies of SARS-CoV-2 /swab, n = 26), AgPOCTs reached sensitivities of 92.3 % or more (range 92.3 %-100 %). Specificity was 100 % for tests I, II (CI95 %: 96.3-100 for both tests) and IV (CI95 %: 96.3-100) and 97 % (CI95 %: 91.5-98.9) for test III. Regarding handling, test I obtained the overall highest scores, while test II was considered to have the most convenient components. Of note, users considered all assays, with the exception of test I, to pose a significant risk for contamination by drips or spills.
Discussion: Besides some differences in sensitivity and handling, all four AgPOCTs showed acceptable performance in high viral load samples. However, due to the significantly lower sensitivity compared to RT-qPCR, a careful consideration of pro and cons of AgPOCT has to be taken into account before clinical implementation.
Keywords: Accuracy; Handling; Rapid antigen test AgPOCT; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Covid-19: Is a second wave hitting Europe? | The BMJ, (n.d.). https://www.bmj.com/content/371/bmj.m4113 (accessed November 19, 2020). - PubMed
-
- Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases, (n.d.). https://www.who.int/publications-detail-redirect/10665-331501 (accessed November 19, 2020).
-
- Liste der Antigentests, (n.d.). https://antigentest.bfarm.de/ords/antigen/r/antigentests-auf-sars-cov-2/liste-der-antigentests?session=21375807250974 (accessed November 24, 2020).
-
- Weitzel T., Legarraga P., Iruretagoyena M., Pizarro G., Vollrath V., Araos R., Munita J.M., Porte L. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. BioRxiv. 2020 doi: 10.1101/2020.05.27.119255. 2020.05.27.119255. - DOI - PMC - PubMed
-
- Comparison of seven commercial SARS-CoV-2 rapid Point-of-Care Antigen tests | medRxiv, (n.d.). https://www.medrxiv.org/content/10.1101/2020.11.12.20230292v1.article-info (accessed November 24, 2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

