Tau assemblies do not behave like independently acting prion-like particles in mouse neural tissue
- PMID: 33712082
- PMCID: PMC7953780
- DOI: 10.1186/s40478-021-01141-6
Tau assemblies do not behave like independently acting prion-like particles in mouse neural tissue
Abstract
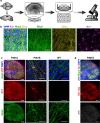
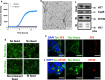
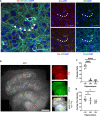
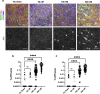
A fundamental property of infectious agents is their particulate nature: infectivity arises from independently-acting particles rather than as a result of collective action. Assemblies of the protein tau can exhibit seeding behaviour, potentially underlying the apparent spread of tau aggregation in many neurodegenerative diseases. Here we ask whether tau assemblies share with classical pathogens the characteristic of particulate behaviour. We used organotypic hippocampal slice cultures from P301S tau transgenic mice in order to precisely control the concentration of extracellular tau assemblies in neural tissue. Whilst untreated slices displayed no overt signs of pathology, exposure to recombinant tau assemblies could result in the formation of intraneuronal, hyperphosphorylated tau structures. However, seeding ability of tau assemblies did not titrate in a one-hit manner in neural tissue. The results suggest that seeding behaviour of tau arises at high concentrations, with implications for the interpretation of high-dose intracranial challenge experiments and the possible contribution of seeded aggregation to human disease.
Keywords: Neurodegeneration; Organotypic hippocampal slice cultures; Prion-like activity; Tau seeded aggregation; Tauopathies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

