Deciphering the functional role of EGR1 in Prostaglandin F2 alpha induced luteal regression applying CRISPR in corpus luteum of buffalo
- PMID: 33712084
- PMCID: PMC7953609
- DOI: 10.1186/s40659-021-00333-7
Deciphering the functional role of EGR1 in Prostaglandin F2 alpha induced luteal regression applying CRISPR in corpus luteum of buffalo
Abstract
Background: PGF2α is essential for the induction of the corpus luteum regression which in turn reduces progesterone production. Early growth response (EGR) proteins are Cys2-His2-type zinc-finger transcription factor that are strongly linked to cellular proliferation, survival and apoptosis. Rapid elevation of EGR1 was observed after luteolytic dose of PGF2α. EGR1 is involved in the transactivation of many genes, including TGFβ1, which plays an important role during luteal regression.
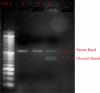
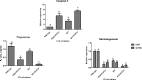
Methods: The current study was conducted in buffalo luteal cells with the aim to better understand the role of EGR1 in transactivation of TGFβ1 during PGF2α induced luteal regression. Luteal cells from mid stage corpus luteum of buffalo were cultured and treated with different doses of PGF2α for different time durations. Relative expression of mRNAs encoding for enzymes within the progesterone biosynthetic pathway (3βHSD, CYP11A1 and StAR); Caspase 3; AKT were analyzed to confirm the occurrence of luteolytic event. To determine if EGR1 is involved in the PGF2α induced luteal regression via induction of TGFβ1 expression, we knocked out the EGR1 gene by using CRISPR/Cas9.
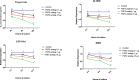
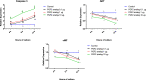
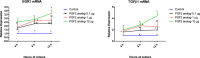
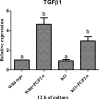
Result: The present experiment determined whether EGR1 protein expression in luteal cells was responsive to PGF2α treatment. Quantification of EGR1 and TGFβ1 mRNA showed significant up regulation in luteal cells of buffalo at 12 h post PGF2α induction. In order to validate the role of PGF2α on stimulating the expression of TGFβ1 by an EGR1 dependent mechanism we knocked out EGR1. The EGR1 ablated luteal cells were stimulated with PGF2α and it was observed that EGR1 KO did not modulate the PGF2α induced expression of TGFβ1. In PGF2α treated EGR1 KO luteal cell, the mRNA expression of Caspase 3 was significantly increased compared to PGF2α treated wild type luteal cells maintained for 12 h. We also studied the influence of EGR1 on steroidogenesis. The EGR1 KO luteal cells with PGF2α treatment showed no substantial difference either in the progesterone concentration or in StAR mRNA expression with PGF2α-treated wild type luteal cells.
Conclusion: These results suggest that EGR1 signaling is not the only factor which plays a role in the regulation of PGF2α induced TGFβ1 signaling for luteolysis.
Keywords: Buffalo; CRISPR/Cas9; Corpus luteum; EGR; Luteolysis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Alila HW, Dowd JP, Corradino RA, Harris WV, Hansel W. Control of progesterone production in small and large bovine luteal cells separated by flow cytometry. JRI. 1988;82:645–655. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

