Intraoperative clonidine to prevent postoperative emergence delirium following sevoflurane anesthesia in pediatric patients: a randomized clinical trial
- PMID: 33712253
- PMCID: PMC9373338
- DOI: 10.1016/j.bjane.2020.12.003
Intraoperative clonidine to prevent postoperative emergence delirium following sevoflurane anesthesia in pediatric patients: a randomized clinical trial
Abstract
Introduction and objective: Emergence Delirium (ED), particularly in children, is characterized by mental confusion, irritability, disorientation, and inconsolable crying. ED prolongs the time required in the Post-Anesthesia Care Unit (PACU) and increases concern and anxiety in parents. The present study aimed to determine the effectiveness and safety of low-dose clonidine in preventing ED in children receiving sevoflurane anesthesia for tonsillectomy/adenotonsillectomy.
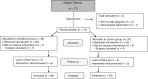
Methods: A randomized, double-blind clinical trial was conducted between November 2013 and January 2014. Sixty-two children aged 2-12 years, scheduled to undergo tonsillectomy/adenotonsillectomy, and classified as American Society of Anesthesiologists (ASA) physical status I/II were included, with 29 being randomized to receive 1 μg.kg-1 clonidine intravenously, and 33 allocated to a control group that received no clonidine. Anesthesia was induced and maintained with sevoflurane. Children with altered state of consciousness, neurological deficit, history of allergy to dipyrone, or receiving other drugs such as preanesthetic agents were excluded from the study. The primary outcome was the presence of ED in the initial 20 minutes in the PACU according to the Pediatric Anesthesia Emergence Delirium (PAED) scale. The Chi-Square test and Fisher's two-tailed exact test were used for statistical analysis, as applicable. Significance level was set at 5%, and Risk Ratios (RR) and their 95% Confidence Intervals (95% CI) were calculated.
Results: The frequency of ED was significantly decreased in the group of children who received clonidine (17.2% vs. 57.6%; RR = 0.30; 95% CI 0.13-0.70; p = 0.001). There was no difference between groups with respect to the frequency of postoperative self-harm (falls and bruises), dislodged catheters, and for most of the other adverse events evaluated.
Conclusions: The use of 1 μg.kg-1 intravenous clonidine during anesthesia induction can effectively reduce the incidence of ED in children undergoing elective tonsillectomy/adenotonsillectomy under general inhalation anesthesia with sevoflurane. CLINICALTRIALS.
Gov identifier: NCT02181543.
Keywords: Anesthesia; Children; Clonidine; Emergence delirium; Psychomotor agitation; Tonsillectomy.
Copyright © 2020. Published by Elsevier Editora Ltda.
Figures
References
-
- Hudek K. Emergence delirium: a nursing perspective. AORN J. 2009;89:509–516. - PubMed
-
- Vlajkovic G.P., Sindjelic R.P. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91. - PubMed
-
- Lapin S.L., Auden S.M., Goldsmith L.J., et al. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatr Anaesth. 1999;9:299–304. - PubMed
-
- Welborn L.G., Hannallah R.S., Norden J.M., Ruttimann U.E., Callan C.M. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg. 1996;83:917–920. - PubMed
-
- Aouad M.T., Nasr V.G. Emergence agitation in children: an update. Curr Opin Anaesthesiol. 2005;18:614–619. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical