Acetyl-CoA and Metabolite Fluxes Regulate White Adipose Tissue Expansion
- PMID: 33712368
- PMCID: PMC8035226
- DOI: 10.1016/j.tem.2021.02.008
Acetyl-CoA and Metabolite Fluxes Regulate White Adipose Tissue Expansion
Abstract
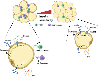
White adipose tissue (WAT) depends on coordinated regulation of transcriptional and metabolic pathways to respond to whole-body energy demands. We highlight metabolites that contribute to biosynthetic reactions for WAT expansion. Recent studies have precisely defined how byproducts of carbohydrate and lipid metabolism affect physiological and endocrine functions in adipocytes. We emphasize the critical emerging roles of short-chain fatty acids (SCFAs) and tricarboxylic acid (TCA) cycle metabolites that connect lipogenesis to WAT energy balance and endocrine functions. These insights address how adipocytes use small molecules generated from central carbon metabolism to measure responses to nutritional stress.
Keywords: adipose tissue; insulin; lipid metabolism; metabolite; microenvironment.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no conflicts of interest.
Figures

References
-
- Ward ZJ et al. (2019) Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 381 (25), 2440–2450. - PubMed
-
- Hammarstedt A et al. (2018) Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol Rev 98 (4), 1911–1941. - PubMed
-
- Caleyachetty R et al. (2017) Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 70 (12), 1429–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

