Atoh7-independent specification of retinal ganglion cell identity
- PMID: 33712461
- PMCID: PMC7954457
- DOI: 10.1126/sciadv.abe4983
Atoh7-independent specification of retinal ganglion cell identity
Abstract
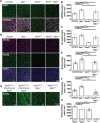
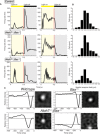
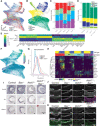
Retinal ganglion cells (RGCs) relay visual information from the eye to the brain. RGCs are the first cell type generated during retinal neurogenesis. Loss of function of the transcription factor Atoh7, expressed in multipotent early neurogenic retinal progenitors leads to a selective and essentially complete loss of RGCs. Therefore, Atoh7 is considered essential for conferring competence on progenitors to generate RGCs. Despite the importance of Atoh7 in RGC specification, we find that inhibiting apoptosis in Atoh7-deficient mice by loss of function of Bax only modestly reduces RGC numbers. Single-cell RNA sequencing of Atoh7;Bax-deficient retinas shows that RGC differentiation is delayed but that the gene expression profile of RGC precursors is grossly normal. Atoh7;Bax-deficient RGCs eventually mature, fire action potentials, and incorporate into retinal circuitry but exhibit severe axonal guidance defects. This study reveals an essential role for Atoh7 in RGC survival and demonstrates Atoh7-dependent and Atoh7-independent mechanisms for RGC specification.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Jarman A. P., Grell E. H., Ackerman L., Jan L. Y., Jan Y. N., Atonal is the proneural gene for Drosophila photoreceptors. Nature 369, 398–400 (1994). - PubMed
-
- Khan K., Logan C. V., Kibbin M. M., Sheridan E., Elçioglu N. H., Yenice O., Parry D. A., Fernandez-Fuentes N., Abdelhamed Z. I. A., Al-Maskari A., Poulter J. A., Mohamed M. D., Carr I. M., Morgan J. E., Jafri H., Raashid Y., Taylor G. R., Johnson C. A., Inglehearn C. F., Toomes C., Ali M., Next generation sequencing identifies mutations in Atonal homolog 7 (ATOH7) in families with global eye developmental defects. Hum. Mol. Genet. 21, 776–783 (2012). - PMC - PubMed
-
- Khor C. C., Ramdas W. D., Vithana E. N., Cornes B. K., Sim X., Tay W.-T., Saw S.-M., Zheng Y., Lavanya R., Wu R., Wang J. J., Mitchell P., Uitterlinden A. G., Rivadeneira F., Teo Y.-Y., Chia K.-S., Seielstad M., Hibberd M., Vingerling J. R., Klaver C. C. W., Jansonius N. M., Tai E.-S., Wong T.-Y., van Duijn C. M., Aung T., Genome-wide association studies in Asians confirm the involvement of ATOH7 and TGFBR3, and further identify CARD10 as a novel locus influencing optic disc area. Hum. Mol. Genet. 20, 1864–1872 (2011). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

