Harnessing the Benefits of Neuroinflammation: Generation of Macrophages/Microglia with Prominent Remyelinating Properties
- PMID: 33712513
- PMCID: PMC8051677
- DOI: 10.1523/JNEUROSCI.1948-20.2021
Harnessing the Benefits of Neuroinflammation: Generation of Macrophages/Microglia with Prominent Remyelinating Properties
Abstract
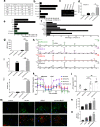
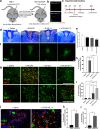
Excessive inflammation within the CNS is injurious, but an immune response is also required for regeneration. Macrophages and microglia adopt different properties depending on their microenvironment, and exposure to IL4 and IL13 has been used to elicit repair. Unexpectedly, while LPS-exposed macrophages and microglia killed neural cells in culture, the addition of LPS to IL4/IL13-treated macrophages and microglia profoundly elevated IL10, repair metabolites, heparin binding epidermal growth factor trophic factor, antioxidants, and matrix-remodeling proteases. In C57BL/6 female mice, the generation of M(LPS/IL4/IL13) macrophages required TLR4 and MyD88 signaling, downstream activation of phosphatidylinositol-3 kinase/mTOR and MAP kinases, and convergence on phospho-CREB, STAT6, and NFE2. Following mouse spinal cord demyelination, local LPS/IL4/IL13 deposition markedly increased lesional phagocytic macrophages/microglia, lactate and heparin binding epidermal growth factor, matrix remodeling, oligodendrogenesis, and remyelination. Our data show that a prominent reparative state of macrophages/microglia is generated by the unexpected integration of pro- and anti-inflammatory activation cues. The results have translational potential, as the LPS/IL4/IL13 mixture could be locally applied to a focal CNS injury to enhance neural regeneration and recovery.SIGNIFICANCE STATEMENT The combination of LPS and regulatory IL4 and IL13 signaling in macrophages and microglia produces a previously unknown and particularly reparative phenotype devoid of pro-inflammatory neurotoxic features. The local administration of LPS/IL4/IL13 into spinal cord lesion elicits profound oligodendrogenesis and remyelination. The careful use of LPS and IL4/IL13 mixture could harness the known benefits of neuroinflammation to enable repair in neurologic insults.
Keywords: macrophages; microglia; neuroinflammation; oligodendrocyte; remyelination.
Copyright © 2021 the authors.
Figures








Similar articles
-
Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis.J Exp Med. 2020 May 4;217(5):e20191390. doi: 10.1084/jem.20191390. J Exp Med. 2020. PMID: 32078678 Free PMC article.
-
IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo.J Neuroinflammation. 2016 Jun 7;13(1):139. doi: 10.1186/s12974-016-0596-5. J Neuroinflammation. 2016. PMID: 27266518 Free PMC article.
-
Induction of autophagy through CLEC4E in combination with TLR4: an innovative strategy to restrict the survival of Mycobacterium tuberculosis.Autophagy. 2020 Jun;16(6):1021-1043. doi: 10.1080/15548627.2019.1658436. Epub 2019 Sep 8. Autophagy. 2020. PMID: 31462144 Free PMC article.
-
Interleukin 4/13 signaling in cardiac regeneration and repair.Am J Physiol Heart Circ Physiol. 2022 Nov 1;323(5):H833-H844. doi: 10.1152/ajpheart.00310.2022. Epub 2022 Sep 23. Am J Physiol Heart Circ Physiol. 2022. PMID: 36149768 Free PMC article. Review.
-
Targeting PKC in microglia to promote remyelination and repair in the CNS.Curr Opin Pharmacol. 2022 Feb;62:103-108. doi: 10.1016/j.coph.2021.11.008. Epub 2021 Dec 26. Curr Opin Pharmacol. 2022. PMID: 34965482 Review.
Cited by
-
Identification of hypoxia-related genes and exploration of their relationship with immune cells in ischemic stroke.Sci Rep. 2023 Jun 29;13(1):10570. doi: 10.1038/s41598-023-37753-2. Sci Rep. 2023. PMID: 37386280 Free PMC article.
-
Astrocyte-derived clusterin disrupts glial physiology to obstruct remyelination in mouse models of demyelinating diseases.Nat Commun. 2024 Sep 6;15(1):7791. doi: 10.1038/s41467-024-52142-7. Nat Commun. 2024. PMID: 39242637 Free PMC article.
-
Thinking outside the box: non-canonical targets in multiple sclerosis.Nat Rev Drug Discov. 2022 Aug;21(8):578-600. doi: 10.1038/s41573-022-00477-5. Epub 2022 Jun 6. Nat Rev Drug Discov. 2022. PMID: 35668103 Free PMC article. Review.
-
Using Human Fetal Neural Stem Cells to Elucidate the Role of the JAK-STAT Cell Signaling Pathway in Oligodendrocyte Differentiation In Vitro.Mol Neurobiol. 2024 Aug;61(8):5738-5753. doi: 10.1007/s12035-024-03928-9. Epub 2024 Jan 16. Mol Neurobiol. 2024. PMID: 38227271
-
Comparison of inflammatory biomarker levels in neurodegenerative proteinopathies: a case-control study.J Neural Transm (Vienna). 2025 Jun;132(6):811-826. doi: 10.1007/s00702-025-02902-6. Epub 2025 Mar 3. J Neural Transm (Vienna). 2025. PMID: 40029428 Free PMC article.
References
-
- Bogie JF, Grajchen E, Wouters E, Garcia Corrales A, Dierckx T, Vanherle S, Mailleux J, Gervois P, Wolfs E, Dehairs J, Van Broeckhoven J, Bowman AP, Lambrichts I, Gustafsson JA, Remaley AT, Mulder M, Swinnen JV, Haidar M, Ellis SR, Ntambi JM, et al. . (2020) Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J Exp Med 217:e20191660. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
