Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis
- PMID: 33712574
- PMCID: PMC7952828
- DOI: 10.1038/s41419-021-03527-9
Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis
Abstract
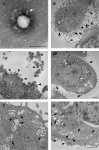
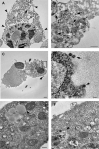
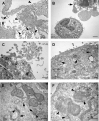
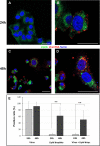
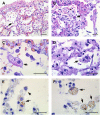
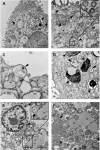
The pathogenesis of SARS-CoV-2 remains to be completely understood, and detailed SARS-CoV-2 cellular cytopathic effects requires definition. We performed a comparative ultrastructural study of SARS-CoV-1 and SARS-CoV-2 infection in Vero E6 cells and in lungs from deceased COVID-19 patients. SARS-CoV-2 induces rapid death associated with profound ultrastructural changes in Vero cells. Type II pneumocytes in lung tissue showed prominent altered features with numerous vacuoles and swollen mitochondria with presence of abundant lipid droplets. The accumulation of lipids was the most striking finding we observed in SARS-CoV-2 infected cells, both in vitro and in the lungs of patients, suggesting that lipids can be involved in SARS-CoV-2 pathogenesis. Considering that in most cases, COVID-19 patients show alteration of blood cholesterol and lipoprotein homeostasis, our findings highlight a peculiar important topic that can suggest new approaches for pharmacological treatment to contrast the pathogenicity of SARS-CoV-2.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO 2020. Coronavirus disease (COVID-19) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio... (2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

