Calcium/Calmodulin-Dependent Protein Kinase II in Cerebrovascular Diseases
- PMID: 33713030
- PMCID: PMC8213567
- DOI: 10.1007/s12975-021-00901-9
Calcium/Calmodulin-Dependent Protein Kinase II in Cerebrovascular Diseases
Abstract
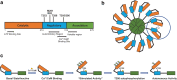
Cerebrovascular disease is the most common life-threatening and debilitating condition that often leads to stroke. The multifunctional calcium/calmodulin-dependent protein kinase II (CaMKII) is a key Ca2+ sensor and an important signaling protein in a variety of biological systems within the brain, heart, and vasculature. In the brain, past stroke-related studies have been mainly focused on the role of CaMKII in ischemic stroke in neurons and established CaMKII as a major mediator of neuronal cell death induced by glutamate excitotoxicity and oxidative stress following ischemic stroke. However, with growing understanding of the importance of neurovascular interactions in cerebrovascular diseases, there are clearly gaps in our understanding of how CaMKII functions in the complex neurovascular biological processes and its contributions to cerebrovascular diseases. Additionally, emerging evidence demonstrates novel regulatory mechanisms of CaMKII and potential roles of the less-studied CaMKII isoforms in the ischemic brain, which has sparked renewed interests in this dynamic kinase family. This review discusses past findings and emerging evidence on CaMKII in several major cerebrovascular dysfunctions including ischemic stroke, hemorrhagic stroke, and vascular dementia, focusing on the unique roles played by CaMKII in the underlying biological processes of neuronal cell death, neuroinflammation, and endothelial barrier dysfunction triggered by stroke. We also highlight exciting new findings, promising therapeutic agents, and future perspectives for CaMKII in cerebrovascular systems.
Keywords: CaMKII; Cerebrovascular disease; Endothelial barrier dysfunction; Neuroinflammation; Neuronal cell death; Stroke; Vascular dementia.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Calcium/Calmodulin-Dependent Kinase (CaMKII) Inhibition Protects Against Purkinje Cell Damage Following CA/CPR in Mice.Mol Neurobiol. 2020 Jan;57(1):150-158. doi: 10.1007/s12035-019-01765-9. Epub 2019 Sep 13. Mol Neurobiol. 2020. PMID: 31520314 Free PMC article.
-
The role of Ca2+-calmodulin stimulated protein kinase II in ischaemic stroke - A potential target for neuroprotective therapies.Neurochem Int. 2017 Jul;107:33-42. doi: 10.1016/j.neuint.2017.01.012. Epub 2017 Jan 31. Neurochem Int. 2017. PMID: 28153786 Review.
-
Vascular CaMKII: heart and brain in your arteries.Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C462-78. doi: 10.1152/ajpcell.00341.2015. Epub 2016 Jun 15. Am J Physiol Cell Physiol. 2016. PMID: 27306369 Free PMC article. Review.
-
CaMKII in cerebral ischemia.Acta Pharmacol Sin. 2011 Jul;32(7):861-72. doi: 10.1038/aps.2011.68. Epub 2011 Jun 20. Acta Pharmacol Sin. 2011. PMID: 21685929 Free PMC article. Review.
-
Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability.J Biol Chem. 2012 Mar 9;287(11):8495-506. doi: 10.1074/jbc.M111.323915. Epub 2012 Jan 17. J Biol Chem. 2012. PMID: 22253441 Free PMC article.
Cited by
-
Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Mediating Function and Dysfunction at Glutamatergic Synapses.Front Mol Neurosci. 2022 Jun 20;15:855752. doi: 10.3389/fnmol.2022.855752. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35795689 Free PMC article. Review.
-
Pyramidal and parvalbumin neurons modulate the process of electroacupuncture stimulation for stroke rehabilitation.iScience. 2024 Apr 9;27(5):109695. doi: 10.1016/j.isci.2024.109695. eCollection 2024 May 17. iScience. 2024. PMID: 38680657 Free PMC article.
-
Unveiling the role of CaMKII in retinal degeneration: from biological mechanism to therapeutic strategies.Cell Biosci. 2024 May 9;14(1):59. doi: 10.1186/s13578-024-01236-2. Cell Biosci. 2024. PMID: 38725013 Free PMC article. Review.
-
Identification of the Beta Subunit Fas1p of Fatty Acid Synthetase as an Interacting Partner of Yeast Calcium/Calmodulin-Dependent Protein Kinase Cmk2p Through Mass Spectrometry Analysis.Appl Biochem Biotechnol. 2024 Oct;196(10):6836-6848. doi: 10.1007/s12010-024-04891-w. Epub 2024 Feb 27. Appl Biochem Biotechnol. 2024. PMID: 38411936
-
An L-type calcium channel blocker nimodipine exerts anti-fibrotic effects by attenuating TGF-β1 induced calcium response in an in vitro model of thyroid eye disease.Eye Vis (Lond). 2024 Sep 6;11(1):37. doi: 10.1186/s40662-024-00401-5. Eye Vis (Lond). 2024. PMID: 39237996 Free PMC article.
References
-
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
-
- Nakamura K, Kamouchi M, Arimura K, Nishimura A, Kuroda J, Ishitsuka K, Tokami H, Sugimori H, Ago T, Kitazono T. Extracellular acidification activates cAMP responsive element binding protein via Na+/H+ exchanger isoform 1-mediated Ca(2)(+) oscillation in central nervous system pericytes. Arterioscler Thromb Vasc Biol. 2012;32(11):2670–2677. doi: 10.1161/ATVBAHA.112.254946. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
