Of membranes and malaria: phospholipid asymmetry in Plasmodium falciparum-infected red blood cells
- PMID: 33713154
- PMCID: PMC11071739
- DOI: 10.1007/s00018-021-03799-6
Of membranes and malaria: phospholipid asymmetry in Plasmodium falciparum-infected red blood cells
Abstract
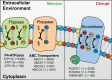
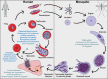
Malaria is a vector-borne parasitic disease with a vast impact on human history, and according to the World Health Organisation, Plasmodium parasites still infect over 200 million people per year. Plasmodium falciparum, the deadliest parasite species, has a remarkable ability to undermine the host immune system and cause life-threatening disease during blood infection. The parasite's host cells, red blood cells (RBCs), generally maintain an asymmetric distribution of phospholipids in the two leaflets of the plasma membrane bilayer. Alterations to this asymmetry, particularly the exposure of phosphatidylserine (PS) in the outer leaflet, can be recognised by phagocytes. Because of the importance of innate immune defence numerous studies have investigated PS exposure in RBCs infected with P. falciparum, but have reached different conclusions. Here we review recent advancements in our understanding of the molecular mechanisms which regulate asymmetry in RBCs, and whether infection with the P. falciparum parasite results in changes to PS exposure. On the balance of evidence, it is likely that membrane asymmetry is disrupted in parasitised RBCs, though some methodological issues need addressing. We discuss the potential causes and consequences of altered asymmetry in parasitised RBCs, particularly for in vivo interactions with the immune system, and the role of host-parasite co-evolution. We also examine the potential asymmetric state of parasite membranes and summarise current knowledge on the parasite proteins, which could regulate asymmetry in these membranes. Finally, we highlight unresolved questions at this time and the need for interdisciplinary approaches to uncover the machinery which enables P. falciparum parasites to hide in mature erythrocytes.
Keywords: Annexin V; Host-Parasite Interactions; Malaria; Phosphatidylserine exposure; Plasmodium falciparum; Red blood cells.
Conflict of interest statement
Not applicable for this study.
Figures

Similar articles
-
Breakdown in membrane asymmetry regulation leads to monocyte recognition of P. falciparum-infected red blood cells.PLoS Pathog. 2021 Feb 18;17(2):e1009259. doi: 10.1371/journal.ppat.1009259. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33600495 Free PMC article.
-
Protein trafficking in Plasmodium falciparum-infected red blood cells.Trends Parasitol. 2004 Dec;20(12):581-9. doi: 10.1016/j.pt.2004.09.008. Trends Parasitol. 2004. PMID: 15522668 Review.
-
Febrile temperature but not proinflammatory cytokines promotes phosphatidylserine expression on Plasmodium falciparum malaria-infected red blood cells during parasite maturation.Cytometry A. 2010 Jun;77(6):515-23. doi: 10.1002/cyto.a.20879. Cytometry A. 2010. PMID: 20191617
-
Characterization of Apicomplexan Amino Acid Transporters (ApiATs) in the Malaria Parasite Plasmodium falciparum.mSphere. 2021 Dec 22;6(6):e0074321. doi: 10.1128/mSphere.00743-21. Epub 2021 Nov 10. mSphere. 2021. PMID: 34756057 Free PMC article.
-
Sticking for a Cause: The Falciparum Malaria Parasites Cytoadherence Paradigm.Front Immunol. 2019 Jun 27;10:1444. doi: 10.3389/fimmu.2019.01444. eCollection 2019. Front Immunol. 2019. PMID: 31316507 Free PMC article. Review.
Cited by
-
2,3-Diphosphoglycerate and the Protective Effect of Pyruvate Kinase Deficiency against Malaria Infection-Exploring the Role of the Red Blood Cell Membrane.Int J Mol Sci. 2023 Jan 10;24(2):1336. doi: 10.3390/ijms24021336. Int J Mol Sci. 2023. PMID: 36674863 Free PMC article.
-
Evaluation of stored red blood cell quality after washing using immune indices.Heliyon. 2024 May 28;10(11):e32056. doi: 10.1016/j.heliyon.2024.e32056. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38882340 Free PMC article.
-
Phosphatidylserine-exposing extracellular vesicles in body fluids are an innate defence against apoptotic mimicry viral pathogens.Nat Microbiol. 2024 Apr;9(4):905-921. doi: 10.1038/s41564-024-01637-6. Epub 2024 Mar 25. Nat Microbiol. 2024. PMID: 38528146 Free PMC article.
-
The enemy within: lipid asymmetry in intracellular parasite-host interactions.Emerg Top Life Sci. 2023 Mar 31;7(1):67-79. doi: 10.1042/ETLS20220089. Emerg Top Life Sci. 2023. PMID: 36820809 Free PMC article. Review.
-
Reversible host cell surface remodelling limits immune recognition and maximizes transmission of Plasmodium falciparum gametocytes.bioRxiv [Preprint]. 2024 Apr 30:2024.04.30.591837. doi: 10.1101/2024.04.30.591837. bioRxiv. 2024. Update in: PLoS Pathog. 2025 May 12;21(5):e1013110. doi: 10.1371/journal.ppat.1013110. PMID: 38746342 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

