Cimetidine-induced androgenic failure causes cell death and changes in actin, EGF and V-ATPase immunoexpression in rat submandibular glands
- PMID: 33713423
- PMCID: PMC8197950
- DOI: 10.1111/joa.13408
Cimetidine-induced androgenic failure causes cell death and changes in actin, EGF and V-ATPase immunoexpression in rat submandibular glands
Abstract
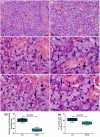
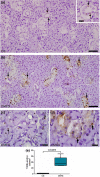
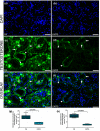
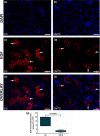
Submandibular gland (SMG) is responsive to androgens via androgen receptor (AR). We verified whether cimetidine induces androgenic dysfunction in SMG, and evaluated the structural integrity, cell death and immunoexpression of actin, EGF and V-ATPase in androgen-deficient SMG. Male rats received cimetidine (CMTG) and control animals (CG) received saline. Granular convoluted tubules (GCTs) diameter and number of acinar cell nuclei were evaluated. TUNEL and immunofluorescence reactions for detection of AR, testosterone, actin, EGF and V-ATPase were quantitatively analysed. In CG, testosterone immunolabelling was detected in acinar and ductal cells cytoplasm. AR-immunolabelled nuclei were observed in acinar cells whereas ductal cells showed AR-immunostained cytoplasm, indicating a non-genomic AR action. In CMTG, the weak testosterone and AR immunoexpression confirmed cimetidine-induced androgenic failure. A high cell death index was correlated with decreased number of acinar cells, GCTs diameter and EGF immunoexpression under androgenic dysfunction. Actin immunofluorescence decreased in the SMG cells, but an increased and diffuse cytoplasmic V-ATPase immunolabelling was observed in striated ducts, suggesting a disruption in the actin-dependent V-ATPase recycling due to androgenic failure. Our findings reinforce the androgenic role in the maintenance of SMG histophysiology, and point to a potential clinical use of cimetidine against androgen-dependent glandular tumour cells.
Keywords: AR; antiandrogen; apoptosis; salivary glands; testosterone.
© 2021 Anatomical Society.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Muscular atrophy, impaired epithelial autophagy and UCHL1 increase in androgen-deficient cauda epididymis.Reproduction. 2020 May;159(6):693-705. doi: 10.1530/REP-19-0462. Reproduction. 2020. PMID: 32191915
-
NF-kB overexpression and decreased immunoexpression of AR in the muscular layer is related to structural damages and apoptosis in cimetidine-treated rat vas deferens.Reprod Biol Endocrinol. 2013 Apr 9;11:29. doi: 10.1186/1477-7827-11-29. Reprod Biol Endocrinol. 2013. PMID: 23570504 Free PMC article.
-
Reduced levels of stromal sex hormone-binding globulin and androgen receptor dysfunction in the sperm storage region of the rat epididymis.Reproduction. 2018 Jun;155(6):467-479. doi: 10.1530/REP-18-0014. Reproduction. 2018. PMID: 29748247
-
Morphological changes and EGF expression in the granular convoluted tubule cells of submandibular glands of Trypanosoma cruzi infected rats.Tissue Cell. 2008 Aug;40(4):293-8. doi: 10.1016/j.tice.2008.02.004. Epub 2008 Apr 10. Tissue Cell. 2008. PMID: 18405932
-
[Submaxillary glands in an endocrine context].J Biol Buccale. 1985 Sep;13(3):185-203. J Biol Buccale. 1985. PMID: 3908453 Review. French.
Cited by
-
Submandibular Gland Pathogenesis Following SARS-CoV-2 Infection and Implications for Xerostomia.Int J Mol Sci. 2024 Jun 21;25(13):6820. doi: 10.3390/ijms25136820. Int J Mol Sci. 2024. PMID: 38999930 Free PMC article.
-
Pharmacological targeting of gastric mucosal barrier with traditional Chinese medications for repairing gastric mucosal injury.Front Pharmacol. 2023 Jun 8;14:1091530. doi: 10.3389/fphar.2023.1091530. eCollection 2023. Front Pharmacol. 2023. PMID: 37361204 Free PMC article. Review.
-
Effects of 4-hexylresorcinol administration on the submandibular glands in a growing rat model.Head Face Med. 2022 Jun 6;18(1):16. doi: 10.1186/s13005-022-00320-7. Head Face Med. 2022. PMID: 35668488 Free PMC article.
References
-
- Adişen, E. , Aral, A. , Aybay, C. & Gürer, M.A. (2008) Salivary epidermal growth factor levels in Behçet's disease and recurrent aphthous stomatitis. Dermatology, 217, 235–240. - PubMed
-
- Adthapanyawanich, K. , Kumchantuek, T. , Nakata, H. , Yamamoto, M. , Wakayama, T. , Nishiuchi, T. et al. (2015) Morphology and gene expression profile of the submandibular gland of androgen‐receptor‐deficient mice. Archives of Oral Biology, 60, 320–332. - PubMed
-
- Ahlner, B.H. , Hagelqvist, E. & Lind, M.G. (1994) Influence on rabbit submandibular gland injury by stimulation or inhibition of gland function during irradiation: Histology and morphometry after 15 gray. Annals of Otology, Rhinology & Laryngology, 103, 125–134. - PubMed
-
- Aldinucci, A. , Bonechi, E. , Manuelli, C. , Nosi, D. , Masini, E. , Passani, M.B. et al. (2016) Histamine regulates actin cytoskeleton in human toll‐like receptor 4‐activated monocyte‐derived dendritic cells tuning CD4+ T lymphocyte response. Journal of Biological Chemistry, 291, 14803–14814. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

