Functional understanding of secondary cell wall cellulose synthases in Populus trichocarpa via the Cas9/gRNA-induced gene knockouts
- PMID: 33713445
- PMCID: PMC8362133
- DOI: 10.1111/nph.17338
Functional understanding of secondary cell wall cellulose synthases in Populus trichocarpa via the Cas9/gRNA-induced gene knockouts
Abstract
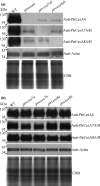
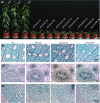
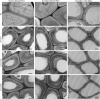
Plant cellulose is synthesized by a large plasma membrane-localized cellulose synthase (CesA) complex. However, an overall functional determination of secondary cell wall (SCW) CesAs is still lacking in trees, especially one based on gene knockouts. Here, the Cas9/gRNA-induced knockouts of PtrCesA4, 7A, 7B, 8A and 8B genes were produced in Populus trichocarpa. Based on anatomical, immunohistochemical and wood composition evidence, we gained a comprehensive understanding of five SCW PtrCesAs at the genetic level. Complete loss of PtrCesA4, 7A/B or 8A/B led to similar morphological abnormalities, indicating similar and nonredundant genetic functions. The absence of the gelatinous (G) layer, one-layer-walled fibres and a 90% decrease in cellulose in these mutant woods revealed that the three classes of SCW PtrCesAs are essential for multilayered SCW structure and wood G-fibre. In addition, the mutant primary and secondary phloem fibres lost the n(G + L)- and G-layers and retained the thicker S-layers (L, lignified; S, secondary). Together with polysaccharide immunolocalization data, these findings suggest differences in the role of SCW PtrCesAs-synthesized cellulose in wood and phloem fibre wall structures. Overall, this functional understanding of the SCW PtrCesAs provides further insights into the impact of lacking cellulose biosynthesis on growth, SCW, wood G-fibre and phloem fibre wall structures in the tree.
Keywords: Cas9; Populus trichocarpa; cellulose synthase (CesA); gRNA; gelatinous layer (G-layer); gene knockout; phloem fibre; secondary cell wall (SCW); tension wood.
© 2021 The Authors New Phytologist © 2021 New Phytologist Foundation.
Figures




References
-
- Abbas M, Peszlen I, Shi R, Kim H, Katahira R, Kafle K, Xiang Z, Huang X, Min D, Mohamadamin Met␣al. 2020. Involvement of CesA4, CesA7‐A/B and CesA8‐A/B in secondary wall formation in Populus trichocarpa wood. Tree Physiology 40: 73–89. - PubMed
-
- Atanassov I, Pittman J, Turner S. 2009. Elucidating the mechanisms of assembly and subunit interaction of the cellulose synthase complex of Arabidopsis secondary cell walls. Journal of Biological Chemistry 284: 3833–3841. - PubMed
-
- Barnett JR, Bonham VA. 2004. Cellulose microfibril angle in the cell wall of wood fibres. Biological Reviews 79: 461–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

