The effect of spike mutations on SARS-CoV-2 neutralization
- PMID: 33713594
- PMCID: PMC7936541
- DOI: 10.1016/j.celrep.2021.108890
The effect of spike mutations on SARS-CoV-2 neutralization
Abstract
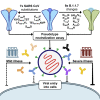
Multiple severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines show protective efficacy, which is most likely mediated by neutralizing antibodies recognizing the viral entry protein, spike. Because new SARS-CoV-2 variants are emerging rapidly, as exemplified by the B.1.1.7, B.1.351, and P.1 lineages, it is critical to understand whether antibody responses induced by infection with the original SARS-CoV-2 virus or current vaccines remain effective. In this study, we evaluate neutralization of a series of mutated spike pseudotypes based on divergence from SARS-CoV and then compare neutralization of the B.1.1.7 spike pseudotype and individual mutations. Spike-specific monoclonal antibody neutralization is reduced dramatically; in contrast, polyclonal antibodies from individuals infected in early 2020 remain active against most mutated spike pseudotypes, but potency is reduced in a minority of samples. This work highlights that changes in SARS-CoV-2 spike can alter neutralization sensitivity and underlines the need for effective real-time monitoring of emerging mutations and their effect on vaccine efficacy.
Keywords: B.1.1.7; SARS-CoV-2; antibodies; immune escape; neutralization; serology; variant.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Amsterdam UMC submitted a patent application on SARS-CoV-2 monoclonal antibodies, some of which were used in this study.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

