Clinical prognostic factors in advanced epithelioid haemangioendothelioma: a retrospective case series analysis within the Italian Rare Cancers Network
- PMID: 33714008
- PMCID: PMC7957151
- DOI: 10.1016/j.esmoop.2021.100083
Clinical prognostic factors in advanced epithelioid haemangioendothelioma: a retrospective case series analysis within the Italian Rare Cancers Network
Abstract
Background: This multicentric, retrospective study conducted within the Italian Rare Cancer Network describes clinical features and explores their possible prognostic relevance in patients with advanced epithelioid haemangioendothelioma (EHE) started on surveillance.
Patients and methods: We collected data on adult patients with molecularly confirmed, advanced EHE consecutively referred at five sarcoma reference centres between January 2010 and June 2018, with no evidence of progressive disease (PD) and started on surveillance. Overall survival (OS) and progression-free survival (PFS) univariable and multivariable Cox analyses were performed. In the latter, due to the low number of cases and events, penalized likelihood was applied, and variable selection was performed using a random forest model.
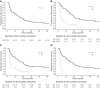
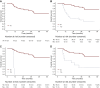
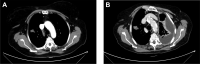
Results: Sixty-seven patients were included. With a median follow-up of 50.2 months, 51 (76%) patients developed PD and 16 (24%) remained stable. PD at treatment start did not meet RECIST version 1.1 in 15/51 (29%) patients. The 3-year PFS and OS were 25.4% and 71.1%, respectively, in the whole population. Tumour-related pain (TRP) was the most common baseline symptom (32.8%), followed by temperature (20.9%), fatigue (17.9%), and weight loss (16.4%). Baseline TRP (P = 0.0002), development of TRP during follow-up (P = 0.005), baseline temperature (P = 0.002), and development of fatigue during follow-up (P = 0.007) were associated with a significantly worst PFS. An association between baseline TRP (P < 0.0001), development of TRP during follow-up (P = 0.0009), evidence of baseline serosal effusion (P = 0.121), and OS was recorded.
Conclusion: Because of the poor outcome observed in EHE patients presenting with serosal effusion, TRP, temperature, or serosal effusion, upfront treatment in this subgroup could be considered.
Keywords: epithelioid haemangioendothelioma; outcome; prognostic factors; serosal effusion; surveillance; symptoms.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure AMF has received institutional research funding from Advenchen, Amgen Dompè, Bayer, Epizyme, Eli Lilly, Daiichi Sankyo, Karyopharm, Novartis, Pfizer, PharmaMar, SpringWorks Therapeutics; travel coverage by PharmaMar. BV has received institutional research grants from Eli Lilly, Novartis, PharmaMar; honoraria for advisory board participation from Eisai, Eli Lilly, Novartis, PharmaMar and Abbot; testimony fee from Abbot; research funding for clinical studies (institutional) from PharmaMar, Eli Lilly, and Novartis. AB has received consultancy and advisory board fee from Eli Lilly, Roche, Eisai; travel grants from PharmaMar, Ipsen. NS has received institutional research funding from Advenchen, Amgen Dompè, Bayer, Epizyme, Eli Lilly, Daiichi Sankyo, Karyopharm, Novartis, Pfizer, PharmaMar, SpringWorks Therapeutics. GG has received research grant from PharmaMar and Bayer; honoraria for advisory board from Lilly, PharmaMar, Novartis, Merck, Bayer, EISAI. PGC received honoraria for speaker, consultancy, or advisory role from Bayer, Deciphera, Eisai, Eli Lilly, Pfizer. His Unit received funds from Advenchen Laboratories, Amgen Dompé, AROG Pharmaceuticals, Bayer, Blueprint Medicines, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly, Epizyme Inc, Glaxo, Karyopharm Pharmaceuticals, Novartis, Pfizer, PharmaMar. SS has received honoraria from Adaptimmune, Bayer, Epizyme, Eli Lilly, Daiichi Sankyo, Deciphera, Immune Design, Karyopharm, Maxivax, PharmaMar, Takeda; institutional research funding from Advenchen, Amgen Dompè, Bayer, Epizyme, Eli Lilly, Daiichi Sankyo, Karyopharm, Novartis, Pfizer, PharmaMar, SpringWorks Therapeutics. All other authors have declared no conflicts of interest.
Figures
References
-
- Weiss S.W., Enzinger F.M. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50(5):970–981. - PubMed
-
- Tanas M.R., Sboner A., Oliveira A.M. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3(98):98ra82. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

