PGC-1α alleviates mitochondrial dysfunction via TFEB-mediated autophagy in cisplatin-induced acute kidney injury
- PMID: 33714196
- PMCID: PMC8034953
- DOI: 10.18632/aging.202653
PGC-1α alleviates mitochondrial dysfunction via TFEB-mediated autophagy in cisplatin-induced acute kidney injury
Abstract
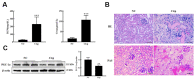
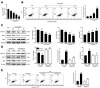
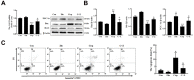
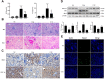
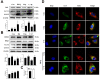
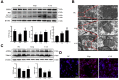
Because of the key role of impaired mitochondria in the progression of acute kidney injury (AKI), it is striking that peroxisome proliferator γ coactivator 1-α (PGC-1α), a transcriptional coactivator of genes involved in mitochondrial biogenesis and autophagy, protects from kidney injury. However, the specific mechanism involved in PGC-1α-mediated autophagy remains elusive. In vivo, along with the severe kidney damage, the expression of PGC-1α was decreased in cisplatin-induced AKI mice. Conversely, PGC-1α activator (ZLN005) administration could alleviate kidney injury. Consistently, in vitro overexpression of PGC-1α or ZLN005 treatment inhibited cell apoptosis and mitochondrial dysfunction induced by cisplatin. Moreover, ZLN005 treatment increased the expression of LC3-II and co-localization between LC3 and mitochondria, suggesting that the mitophagy was activated. Furthermore, PGC-1α-mediated the activation of mitophagy was reliant on the increased expression of TFEB, and the protective effects were abrogated in TFEB-knockdown cells. These data suggest that the activation of PGC-1α could alleviate mitochondrial dysfunction and kidney injury in AKI mice via TFEB-mediated autophagy.
Keywords: PGC-1α; TFEB; acute kidney injury; autophagy; mitochondrial dysfunction.
Conflict of interest statement
Figures



References
-
- Neamatallah T, El-Shitany NA, Abbas AT, Ali SS, Eid BG. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 2018; 9:3743–54. 10.1039/c8fo00653a - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

