Pernio (Chilblains), SARS-CoV-2, and COVID Toes Unified Through Cutaneous and Systemic Mechanisms
- PMID: 33714595
- PMCID: PMC7826004
- DOI: 10.1016/j.mayocp.2021.01.009
Pernio (Chilblains), SARS-CoV-2, and COVID Toes Unified Through Cutaneous and Systemic Mechanisms
Abstract
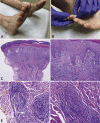
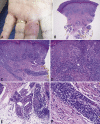
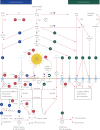
Pernio or chilblains is characterized by erythema and swelling at acral sites (eg, toes and fingers), typically triggered by cold exposure. Clinical and histopathologic features of pernio are well described, but the pathogenesis is not entirely understood; vasospasm and a type I interferon (IFN-I) immune response are likely involved. During the coronavirus disease 2019 (COVID-19) pandemic, dermatologists have observed an increase in pernio-like acral eruptions. Direct causality of pernio due to COVID-19 has not been established in many cases because of inconsistent testing methods (often negative results) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, a form of COVID-19‒associated pernio (also called COVID toes) is probable because of increased occurrence, frequently in young patients with no cold exposure or a history of pernio, and reports of skin biopsies with positive SARS-CoV-2 immunohistochemistry. PubMed was searched between January 1, 2020, and December 31, 2020 for publications using the following keywords: pernio, chilblain, and acral COVID-19. On the basis of our review of the published literature, we speculate that several unifying cutaneous and systemic mechanisms may explain COVID-19‒associated pernio: (1) SARS-CoV-2 cell infection occurs through the cellular receptor angiotensin-converting enzyme 2 mediated by transmembrane protease serine 2, subsequently affecting the renin-angiotensin-aldosterone system with an increase in the vasoconstricting, pro-inflammatory, and prothrombotic angiotensin II pathway. (2) Severe acute respiratory syndrome coronavirus 2 cell infection triggers an immune response with robust IFN-I release in patients predisposed to COVID-19‒associated pernio. (3) Age and sex discrepancies correlated with COVID-19 severity and manifestations, including pernio as a sign of mild disease, are likely explained by age-related immune and vascular differences influenced by sex hormones and genetics, which affect susceptibility to viral cellular infection, the renin-angiotensin-aldosterone system balance, and the IFN-I response.
Copyright © 2021 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Florida COVID-19 response Florida Health website. https://floridahealthcovid19.gov/#latest-stats
-
- Pernio Wiktionary website. https://en.wiktionary.org/wiki/pernio
-
- Cappel J.A., Wetter D.A. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89(2):207–215. - PubMed
-
- Su W.P., Perniciaro C., Rogers R.S., III, White J.W., Jr. Chilblain lupus erythematosus (lupus pernio): clinical review of the Mayo Clinic experience and proposal of diagnostic criteria. Cutis. 1994;54(6):395–399. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

