Human splenic myeloid derived suppressor cells: Phenotypic and clustering analysis
- PMID: 33714729
- PMCID: PMC8077751
- DOI: 10.1016/j.cellimm.2021.104317
Human splenic myeloid derived suppressor cells: Phenotypic and clustering analysis
Abstract
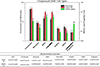
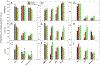
Myeloid derived suppressor cells (MDSCs) can be subset into monocytic (M-), granulocytic (G-) or polymorphonuclear (PMN-), and immature (i-) or early MDSCs and have a role in many disease states. In cancer patients, the frequencies of MDSCs can positively correlate with stage, grade, and survival. Most clinical studies into MDSCs have been undertaken with peripheral blood (PB); however, in the present studies, we uniquely examined MDSCs in the spleens and PB from patients with gastrointestinal cancers. In our studies, MDSCs were rigorously subset using the following markers: Lineage (LIN) (CD3, CD19 and CD56), human leukocyte antigen (HLA)-DR, CD11b, CD14, CD15, CD33, CD34, CD45, and CD16. We observed a significantly higher frequency of PMN- and M-MDSCs in the PB of cancer patients as compared to their spleens. Expression of the T-cell suppressive enzymes arginase (ARG1) and inducible nitric oxide synthase (i-NOS) were higher on all MDSC subsets for both cancer patients PB and spleen cells as compared to MDSCs from the PB of normal donors. Similar findings for the activation markers lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), program death ligand 1 (PD-L1) and program cell death protein 1 (PD-1) were observed. Interestingly, the total MDSC cell number exported to clustering analyses was similar between all sample types; however, clustering analyses of these MDSCs, using these markers, uniquely documented novel subsets of PMN-, M- and i-MDSCs. In summary, we report a comparison of splenic MDSC frequency, subtypes, and functionality in cancer patients to their PB by clustering and cytometric analyses.
Keywords: Cancer patient peripheral blood; Cancer patient spleen; Flow cytometry; MDSC; SPADE.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
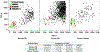



References
-
- Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL, Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins, Journal of immunology, 190 (2013) 794–804. - PubMed
-
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ, Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy, Cancer Immunol Immunother, 58 (2009) 49–59. - PMC - PubMed
-
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW, Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13, Cancer Immunol Immunother, 60 (2011) 1419–1430. - PMC - PubMed
-
- Young MRI, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL, Petruzzelli GJ, Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells, International Journal of Cancer, 74 (1997) 69–74. - PubMed
-
- Slavin S, Strober S, Induction of allograft tolerance after total lymphoid irradiation (TLI): development of suppressor cells of the mixed leukocyte reaction (MLR) Journal of immunology, 123 (1979) 942–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

