The current landscape of the FDA approved companion diagnostics
- PMID: 33714919
- PMCID: PMC7957094
- DOI: 10.1016/j.tranon.2021.101063
The current landscape of the FDA approved companion diagnostics
Abstract
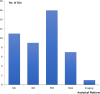
Predictive biomarker is an important element in the realization of precision medicine and with the introduction of the drug-diagnostic codevelopment model, the number of regulatory approved companion diagnostics (CDx) have steadily increased. This short perspective is based on an analysis of the FDA List of Cleared or Approved Companion Diagnostic Devices and focus on the biomarkers, drugs, clinical indications, analytical platforms, regulatory paths and status related to the different assays. By the end of 2020, the total number of CDx assays approved by the FDA had reached 44. These assays are almost exclusively linked to different hematological and oncological drugs. Without an accurate and reliable CDx assay these drugs will lose their value. The analytical platforms are diverse and cover technologies like immunohistochemistry, in situ hybridization, polymerase chain reaction, next generation sequencing, and imaging. CDx assays are high risk devices and the regulatory path almost exclusively requires submission of a Premarket Application (PMA); however, a relatively large group of the CDx assays is PMA approved Laboratory Developed Test.
Keywords: Biomarkers; Companion diagnostics; Laboratory developed test; Personalized medicine; Precision medicine; Premarket application.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest Jan Trøst Jørgensen has worked as a consultant for Agilent Technologies, Euro Diagnostica, Oncology Venture, Azanta, Alligator Biosciences, and Leo Pharma and has given lectures at meetings sponsored by AstraZeneca, Merck Sharp & Dohme, and Roche.
Figures

References
-
- Hayes D.F. HER2 and Breast Cancer - A Phenomenal Success Story. N. Engl. J. Med. 2019;381:1284–1286. - PubMed
-
- Sawyers C.L. Herceptin: a first assault on oncogenes that launched a revolution. Cell. 2019;179:8–12. - PubMed
-
- Jørgensen J.T. Companion and complementary diagnostics: clinical and regulatory perspectives. Trends Cancer. 2016;2:706–712. - PubMed
-
- FDA. List of cleared or approved companion diagnostic devices (In Vitro and Imaging Tools). Update: 11/16/2020. ( https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-ap... ). Accessed January 12, 2021
-
- FDA. Guidance for industry and food and drug administration staff. In vitro companion diagnostic devices. 2014 ( https://www.fda.gov/media/81309/download ). Accessed January 14, 2021
LinkOut - more resources
Full Text Sources
Other Literature Sources

