Protective effects of ginseng stem-leaf saponins on D-galactose-induced reproductive injury in male mice
- PMID: 33714944
- PMCID: PMC8034965
- DOI: 10.18632/aging.202709
Protective effects of ginseng stem-leaf saponins on D-galactose-induced reproductive injury in male mice
Abstract
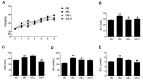
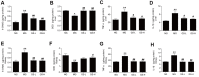
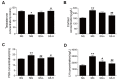
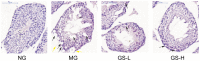
Panax ginseng is a perennial plant in the Araliaceae family. In this study, we investigated the protective effects of ginseng stem-leaf saponins (GSLS) isolated from P. ginseng against D-galactose-induced reproductive function decline, oxidative stress, and inflammatory response. Reproductive injuries were induced in mice via the subcutaneous injection of D-galactose (300 mg/kg) for six weeks. The mice were then treated with GSLS by intragastric administration. GSLS inhibited markers of oxidative stress and inflammatory cytokines induced by D-galactose in serum, liver and kidney, whereas GSLS increased the activities of antioxidant enzymes. Compared to the mice treated only with D-galactose, GSLS treatment significantly increased the average path velocity, straight line velocity, curvilinear velocity, and amplitude of the lateral head displacement of mouse sperm. Meanwhile, GSLS significantly increased the testosterone level and reduced the cortisol, FSH, and LH levels. Histopathological examination revealed alterations in the number and the arrangement of spermatogenic cells in the seminiferous tubules of the mice in the GSLS group. GSLS treatment suppressed MAPKs pathway activation in testes. These results suggest that GSLS can attenuate D-galactose-induced oxidative stress and inflammatory response in serum, liver and kidney, and ameliorate reproductive damage by inhibiting MAPKs signaling pathway.
Keywords: D-galactose; ginseng stem-leaf saponins; inflammatory response; oxidative stress; reproductive function.
Conflict of interest statement
Figures



References
-
- Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC study team (Avon longitudinal study of pregnancy and childhood). Hum Reprod. 2000; 15:1703–08. 10.1093/humrep/15.8.1703 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

