Age cohorts stratified according to age-distributions of COVID-19 morbidity statistics identify uniquely age-dependent CD3+CD8+ T-cell lymphocytopenia in COVID-19 patients without comorbidities on admission
- PMID: 33714947
- PMCID: PMC8034949
- DOI: 10.18632/aging.202691
Age cohorts stratified according to age-distributions of COVID-19 morbidity statistics identify uniquely age-dependent CD3+CD8+ T-cell lymphocytopenia in COVID-19 patients without comorbidities on admission
Abstract
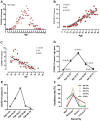
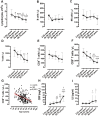
If age boundaries are arbitrarily or roughly defined, age-related analyses can result in questionable findings. Here, we aimed to delineate the uniquely age-dependent immune features of coronavirus disease 2019 (COVID-19) in a retrospective study of 447 patients, stratified according to age distributions of COVID-19 morbidity statistics into well-defined age-cohorts (2-25y, 26-38y, 39-57y, 58-68y, and 69-79y). Age-dependent susceptibilities and severities of the disease were observed in COVID-19 patients. A comparison of the lymphocyte counts among the five age-groups indicated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection led to age-dependent lymphopenia. Among the lymphocyte subsets, the CD8+ T cell count alone was significantly and age-dependently decreased (520, 385, 320, 172, and 139 n/μl in the five age-groups, respectively). In contrast, the CD4+ T cell, B cell, and natural killer cell counts did not differ among age-cohorts. Age and CD8+ T cell counts (r=‒0.435, p<0.0001) were negatively correlated in COVID-19 patients. Moreover, SARS-CoV-2 infection age-dependently increased the plasma C-reactive protein concentrations (2.0, 5.0, 9.0, 11.6, and 36.1 mg/L in the five age-groups, respectively). These findings can be used to elucidate the role of CD8+ T cells in age-related pathogenesis and to help develop therapeutic strategies for COVID-19.
Keywords: C-reactive protein; CD8+ T cells; COVID-19; age-dependent immune features; coronavirus disease 2019.
Conflict of interest statement
Figures
Similar articles
-
Viral loads, lymphocyte subsets and cytokines in asymptomatic, mildly and critical symptomatic patients with SARS-CoV-2 infection: a retrospective study.Virol J. 2021 Jun 12;18(1):126. doi: 10.1186/s12985-021-01597-x. Virol J. 2021. PMID: 34118952 Free PMC article.
-
Clinical and Immune Features of Hospitalized Pediatric Patients With Coronavirus Disease 2019 (COVID-19) in Wuhan, China.JAMA Netw Open. 2020 Jun 1;3(6):e2010895. doi: 10.1001/jamanetworkopen.2020.10895. JAMA Netw Open. 2020. PMID: 32492165 Free PMC article.
-
Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells.Clin Infect Dis. 2020 Nov 19;71(16):2150-2157. doi: 10.1093/cid/ciaa630. Clin Infect Dis. 2020. PMID: 32442287 Free PMC article.
-
The contributory role of lymphocyte subsets, pathophysiology of lymphopenia and its implication as prognostic and therapeutic opportunity in COVID-19.Int Immunopharmacol. 2021 Jun;95:107586. doi: 10.1016/j.intimp.2021.107586. Epub 2021 Mar 18. Int Immunopharmacol. 2021. PMID: 33765611 Free PMC article. Review.
-
Peripheral T cell lymphopenia in COVID-19: potential mechanisms and impact.Immunother Adv. 2021 Jul 2;1(1):ltab015. doi: 10.1093/immadv/ltab015. eCollection 2021 Jan. Immunother Adv. 2021. PMID: 35965490 Free PMC article. Review.
Cited by
-
Zebrafish models of COVID-19.FEMS Microbiol Rev. 2023 Jan 16;47(1):fuac042. doi: 10.1093/femsre/fuac042. FEMS Microbiol Rev. 2023. PMID: 36323404 Free PMC article. Review.
-
The bullwhip effect, T-cell telomeres, and SARS-CoV-2.Lancet Healthy Longev. 2022 Oct;3(10):e715-e721. doi: 10.1016/S2666-7568(22)00190-8. Lancet Healthy Longev. 2022. PMID: 36202131 Free PMC article. Review.
-
Inflammation/Coagulopathy/Immunology Responsive Index Predicts Poor COVID-19 Prognosis.Front Cell Infect Microbiol. 2022 Mar 4;12:807332. doi: 10.3389/fcimb.2022.807332. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35310845 Free PMC article.
-
Inflammation/coagulopathy/fibrinolysis: Dynamic indicators of COVID-19 progression in patients with moderate COVID-19 in Wenzhou, China.Clin Immunol. 2021 Nov;232:108852. doi: 10.1016/j.clim.2021.108852. Epub 2021 Sep 11. Clin Immunol. 2021. PMID: 34520860 Free PMC article.
-
Prevalence of Hypertension and Diabetes in Severe COVID-19: A Cross-Sectional Study from Single Center, Kabul.Infect Drug Resist. 2024 Apr 30;17:1677-1683. doi: 10.2147/IDR.S451114. eCollection 2024. Infect Drug Resist. 2024. PMID: 38707991 Free PMC article.
References
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, et al.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395:1054–62. 10.1016/S0140-6736(20)30566-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

