Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG
- PMID: 33714955
- PMCID: PMC8034903
- DOI: 10.18632/aging.202677
Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG
Abstract
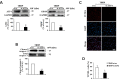
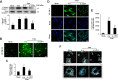
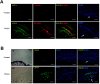
Primary open angle glaucoma (POAG) is the leading cause of irreversible blindness. Dysfunction of the trabecular meshwork (TM), resulting in decreased outflow of aqueous humor and increased intraocular pressure (IOP), plays an important role in the pathogenesis of POAG. However, the underlying mechanisms still remain unclear. In this study, we demonstrated that the eIF2-α/ATF4/CHOP branch of unfolded protein response (UPR) was activated in human trabecular meshwork cells (HTMCs) upon tert-butyl hydroperoxide (TBHP) exposure. Inhibition of ATF4 ameliorated TBHP-induced apoptosis and inflammatory cytokine production, while ectopic expression of ATF4 increased the expression of endothelial leukocyte adhesion molecule (ELAM)-1 and IL-8 in HTMCs. Furthermore, we found that ATF4 inhibition reduced tunicamycin-induced caspase-3 activation, ROS production, ELAM-1 expression, and HTMCs phagocytosis impairment. By an in vivo study in mice, we showed that overexpression of ATF4 in the TM induced C/EBP homologous protein (CHOP) expression and TM cells apoptosis, contributing to inflammatory cytokine production, and probably IOP elevation. More importantly, upregulation of ATF4 and CHOP, and colocalization of ATF4 with ELAM-1 were found in the TM of POAG patients. These results suggest that ATF4 is a critical mediator of oxidative stress and ER stress-induced TM cell dysfunction and apoptosis in POAG.
Keywords: activating transcription factor 4; endoplasmic reticulum stress; oxidative stress; primary open angle glaucoma; trabecular meshwork.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

