Implications of microscale lung damage for COVID-19 pulmonary ventilation dynamics: A narrative review
- PMID: 33716059
- PMCID: PMC7946865
- DOI: 10.1016/j.lfs.2021.119341
Implications of microscale lung damage for COVID-19 pulmonary ventilation dynamics: A narrative review
Abstract
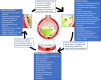
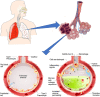
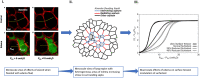
The COVID-19 pandemic surges on as vast research is produced to study the novel SARS-CoV-2 virus and the disease state it induces. Still, little is known about the impact of COVID-19-induced microscale damage in the lung on global lung dynamics. This review summarizes the key histological features of SARS-CoV-2 infected alveoli and links the findings to structural tissue changes and surfactant dysfunction affecting tissue mechanical behavior similar to changes seen in other lung injury. Along with typical findings of diffuse alveolar damage affecting the interstitium of the alveolar walls and blood-gas barrier in the alveolar airspace, COVID-19 can cause extensive microangiopathy in alveolar capillaries that further contribute to mechanical changes in the tissues and may differentiate it from previously studied infectious lung injury. Understanding microlevel damage impact on tissue mechanics allows for better understanding of macroscale respiratory dynamics. Knowledge gained from studies into the relationship between microscale and macroscale lung mechanics can allow for optimized treatments to improve patient outcomes in case of COVID-19 and future respiratory-spread pandemics.
Keywords: Coronavirus infection; Diffuse alveolar damage; Fibrotic lesions; Microangiopathy; Micromechanics; Surfactant dysfunction.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare no competing interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

