Identification of regulated proteins by resveratrol in glutamate-induced cortical injury of newborn rats
- PMID: 33716268
- PMCID: PMC8111349
- DOI: 10.1292/jvms.21-0013
Identification of regulated proteins by resveratrol in glutamate-induced cortical injury of newborn rats
Abstract
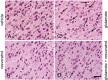
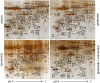
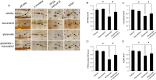
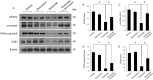
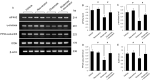
Glutamate induces neuronal damage by generating oxidative stress and neurotoxicities. The neurological damage caused by glutamate is more severe during brain development in newborns than in adults. Resveratrol is naturally present in a variety of fruits and medicinal plants and exerts a neuroprotective effect against brain damage. The goal of this study was to evaluate the neuroprotective effects of resveratrol and to identify changed proteins in response to resveratrol treatment during glutamate-induced neonatal cortical damage. Sprague-Dawley rat pups (7 days old) were randomly divided into vehicle, resveratrol, glutamate, and glutamate and resveratrol groups. The animals were intraperitoneally injected with glutamate (10 mg/kg) and/or resveratrol (20 mg/kg) and their brain tissue was collected 4 hr after drug administration. Glutamate exposure caused severe histopathological changes, while resveratrol attenuated this damage. We identified regulated proteins by resveratrol in glutamate-induced cortical damaged tissue using two-dimensional gel electrophoresis and mass spectrometry. Among identified proteins, we focused on eukaryotic initiation factor 4A2, γ-enolase, protein phosphatase 2A subunit B, and isocitrate dehydrogenase. These proteins decreased in the glutamate-treated group, whereas the combination treatment of glutamate and resveratrol attenuated these protein reductions. These proteins are anti-oxidant proteins and anti-apoptotic proteins. These results suggest that glutamate induces brain cortical damage in newborns; resveratrol exerts a neuroprotective effect by controlling expression of various proteins with anti-oxidant and anti-apoptotic functions.
Keywords: cerebral cortex; glutamate; neonate; proteomics; resveratrol.
Conflict of interest statement
The authors declare no competing financial interests
Figures
Similar articles
-
Proteomic identification of proteins differentially expressed in response to resveratrol treatment in middle cerebral artery occlusion stroke model.J Vet Med Sci. 2014 Oct;76(10):1367-74. doi: 10.1292/jvms.14-0169. Epub 2014 Jul 7. J Vet Med Sci. 2014. PMID: 24998396 Free PMC article.
-
Identification of proteins differentially expressed by glutamate treatment in cerebral cortex of neonatal rats.Lab Anim Res. 2019 Nov 26;35:24. doi: 10.1186/s42826-019-0026-9. eCollection 2019. Lab Anim Res. 2019. PMID: 32257912 Free PMC article.
-
Identification of regulated proteins by epigallocatechin gallate treatment in an ischemic cerebral cortex animal model: a proteomics approach.J Vet Med Sci. 2021 Jun 9;83(6):916-926. doi: 10.1292/jvms.21-0089. Epub 2021 Apr 21. J Vet Med Sci. 2021. PMID: 33883340 Free PMC article.
-
Quercetin alleviates the injury-induced decrease of protein phosphatase 2A subunit B in cerebral ischemic animal model and glutamate-exposed HT22 cells.J Vet Med Sci. 2019 Jul 19;81(7):1047-1054. doi: 10.1292/jvms.19-0094. Epub 2019 May 16. J Vet Med Sci. 2019. PMID: 31092742 Free PMC article.
-
Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats.Neurochem Int. 2010 Feb;56(3):495-500. doi: 10.1016/j.neuint.2009.12.009. Epub 2009 Dec 21. Neurochem Int. 2010. PMID: 20026214
Cited by
-
Investigation of the Effects of Monosodium Glutamate on the Embryonic Development of the Eye in Chickens.Vet Sci. 2023 Jan 30;10(2):99. doi: 10.3390/vetsci10020099. Vet Sci. 2023. PMID: 36851403 Free PMC article.
-
Lens Proteomics Provide Novel Clues for Cataractogenesis: Original Investigation and a Broad Literature Survey.J Clin Med. 2025 Jul 4;14(13):4737. doi: 10.3390/jcm14134737. J Clin Med. 2025. PMID: 40649110 Free PMC article.
-
Quercetin attenuated ischemic stroke induced neurodegeneration by modulating glutamatergic and synaptic signaling pathways.Heliyon. 2024 Mar 20;10(7):e28016. doi: 10.1016/j.heliyon.2024.e28016. eCollection 2024 Apr 15. Heliyon. 2024. Retraction in: Heliyon. 2025 Mar 25;11(9):e43266. doi: 10.1016/j.heliyon.2025.e43266. PMID: 38571617 Free PMC article. Retracted.
References
-
- Abdel-Aleem G. A., Khaleel E. F., Mostafa D. G., Elberier L. K.2016. Neuroprotective effect of resveratrol against brain ischemia reperfusion injury in rats entails reduction of DJ-1 protein expression and activation of PI3K/Akt/GSK3b survival pathway. Arch. Physiol. Biochem. 122: 200–213. doi: 10.1080/13813455.2016.1182190 - DOI - PubMed
-
- Benderdour M., Charron G., Comte B., Ayoub R., Beaudry D., Foisy S., Deblois D., Des Rosiers C.2004. Decreased cardiac mitochondrial NADP+-isocitrate dehydrogenase activity and expression: a marker of oxidative stress in hypertrophy development. Am. J. Physiol. Heart Circ. Physiol. 287: H2122–H2131. doi: 10.1152/ajpheart.00378.2004 - DOI - PubMed
-
- Chaparro-Huerta V., Rivera-Cervantes M. C., Flores-Soto M. E., Gómez-Pinedo U., Beas-Zárate C.2005. Proinflammatory cytokines and apoptosis following glutamate-induced excitotoxicity mediated by p38 MAPK in the hippocampus of neonatal rats. J. Neuroimmunol. 165: 53–62. doi: 10.1016/j.jneuroim.2005.04.025 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

